Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Searches and Study Identification
2.2. Inclusion and Exclusion Criteria
2.3. Modeling for Network Meta-Analysis
2.4. Methodological Quality Appraisal
2.5. Primary Outcome: Quality-of-Life Improvement, Standardized Mean Difference
2.6. Secondary Outcome: Risk Difference of Dropout Rates
2.7. Data Extraction, Management and Conversion
2.8. Statistical Analyses
2.9. Sensitivity Analyses
2.10. Publication Bias
3. Results
3.1. Study Identification and Network Model Formation
3.2. Methodological Quality of the Included Studies
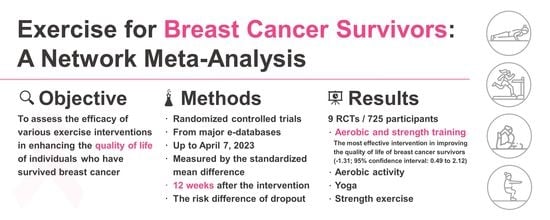
3.3. Primary Outcome: Aerobic and Strength Concurrent Training Most Effective
3.4. Secondary Outcome: Dropout Rates Statistically Similar
3.5. Inconsistency Test
3.6. Sensitivity Analyses
3.7. Publication Bias
4. Discussion
4.1. Main Findings and Clinical Implications
4.2. Significance of the Findings Compared to Existing Literature
4.3. Possible Explanations for the Observed Results
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kern, R.; Correa, S.C.; Scandolara, T.B.; Carla da Silva, J.; Pires, B.R.; Panis, C. Current advances in the diagnosis and personalized treatment of breast cancer: Lessons from tumor biology. Per. Med. 2020, 17, 399–420. [Google Scholar] [CrossRef]
- Cathcart-Rake, E.J.; Ruddy, K.J. Evidence-based guidance for breast cancer survivorship. Hematol. Oncol. Clin. N. Am. 2023, 37, 225–243. [Google Scholar] [CrossRef]
- Soldato, D.; Arecco, L.; Agostinetto, E.; Franzoi, M.A.; Mariamidze, E.; Begijanashvili, S.; Brunetti, N.; Spinaci, S.; Solinas, C.; Vaz-Luis, I.; et al. The future of breast cancer research in the survivorship field. Oncol. Ther. 2023, 11, 199–229. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaglehouse, Y.L.; Poppenberg, J.T.; Brufsky, J.W.; Geramita, E.M.; Zhai, S.; Davis, K.K.; Gibbs, B.B.; Metz, J.; van Londen, G.J. Effects of a personal trainer-led exercise intervention on physical activity, physical function, and quality of life of breast cancer survivors. Breast Cancer 2021, 28, 737–745. [Google Scholar] [CrossRef]
- Muñoz-Gómez, E.; Arnal-Gómez, A.; López Cascón, A.; Espí-López, G.V. Systematic review of aquatic therapeutic exercise efficacy in breast cancer survivors. Support. Care Cancer 2022, 31, 44. [Google Scholar] [CrossRef] [PubMed]
- Ergun, M.; Eyigor, S.; Karaca, B.; Kisim, A.; Uslu, R. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur. J. Cancer Care 2013, 22, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Markozannes, G.; Abar, L.; Balducci, K.; Cariolou, M.; Nanu, N.; Vieira, R.; Anifowoshe, Y.O.; Greenwood, D.C.; Clinton, S.K.; et al. Physical activity and health-related quality of life in women with breast cancer: A meta-analysis. JNCI Cancer Spectr. 2022, 6, pkac072. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Anton, P.M.; Hopkins-Price, P.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; Mocharnuk, R.; McAuley, E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2015, 149, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-Maldonado, A.; Díez-Fernández, D.M.; Esteban-Simón, A.; Rodríguez-Pérez, M.A.; Artés-Rodríguez, E.; Casimiro-Artés, M.A.; Moreno-Martos, H.; Toro-de-Federico, A.; Hachem-Salas, N.; Bartholdy, C.; et al. Effects of a 12-week supervised resistance training program, combined with home-based physical activity, on physical fitness and quality of life in female breast cancer survivors: The EFICAN randomized controlled trial. J. Cancer Surviv. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cramer, H.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G. Yoga and meditation for menopausal symptoms in breast cancer survivors-A randomized controlled trial. Cancer 2015, 121, 2175–2184. [Google Scholar] [CrossRef]
- Kim, S.; Ko, Y.H.; Song, Y.; Kang, M.J.; Lee, H.; Kim, S.H.; Jeon, J.Y.; Cho, Y.U.; Yi, G.; Han, J. Pre-post analysis of a social capital-based exercise adherence intervention for breast cancer survivors with moderate fatigue: A randomized controlled trial. Support Care Cancer 2020, 28, 5281–5289. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, A.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking Network Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-11 (accessed on 25 February 2023).
- Su, X.; McDonough, D.J.; Chu, H.; Quan, M.; Gao, Z. Application of network meta-analysis in the field of physical activity and health promotion. J. Sport. Health Sci. 2020, 9, 511–520. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Tsai, I.C. INPLASY202340007 Comparative Effectiveness of Different Exercises for Improving Quality of Life in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Available online: http://doi.org/10.37766/inplasy2023.4.0007 (accessed on 5 April 2023).
- Mikkelsen, M.K.; Juhl, C.B.; Lund, C.M.; Jarden, M.; Vinther, A.; Nielsen, D.L. The effect of exercise-based interventions on health-related quality of life and physical function in older patients with cancer receiving medical antineoplastic treatments: A systematic review. Eur. Rev. Aging Phys. Act. 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Bula, A.; Tatar, K.; Wysocka, R.; Chyrek, K.; Piejko, L.; Nawrat-Szołtysik, A.; Polak, A. Effect of physical activity on static and dynamic postural balance in women treated for breast cancer: A systematic review. Int. J. Environ. Res. Public. Health 2023, 20, 3722. [Google Scholar] [CrossRef]
- Luo, X.C.; Liu, J.; Fu, J.; Yin, H.Y.; Shen, L.; Liu, M.L.; Lan, L.; Ying, J.; Qiao, X.L.; Tang, C.Z.; et al. Effect of Tai Chi Chuan in breast cancer patients: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 607. [Google Scholar] [CrossRef]
- O’Neill, M.; Samaroo, D.; Lopez, C.; Tomlinson, G.; Santa Mina, D.; Sabiston, C.; Culos-Reed, N.; Alibhai, S.M.H. The effect of yoga interventions on cancer-related fatigue and quality of life for women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Integr. Cancer Ther. 2020, 19, 1534735420959882. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bilbao, T.; Alonso-Dueñas, M.; Peinado, A.B.; San Juan, A.F. Effects of combined interventions of exercise and diet or exercise and supplementation on breast cancer patients: A systematic review. Nutrients 2023, 15, 1013. [Google Scholar] [CrossRef]
- Toohey, K.; Chapman, M.; Rushby, A.M.; Urban, K.; Ingham, G.; Singh, B. The effects of physical exercise in the palliative care phase for people with advanced cancer: A systematic review with meta-analysis. J. Cancer Surviv. 2022, 17, 399–415. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Saco-Ledo, G.; Santos-Lozano, A.; Morales, J.S.; Castillo-García, A.; Simpson, R.J.; Lucia, A.; Fiuza-Luces, C. Exercise training and natural killer cells in cancer survivors: Current evidence and research gaps based on a systematic review and meta-analysis. Sports Med. Open. 2022, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Matsuoka, Y.J.; Ochi, E. High-intensity interval training in breast cancer survivors: A systematic review. BMC Cancer 2021, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, F.; Westerink, N.L.; Berendsen, A.J.; Walenkamp, A.M.E.; De Greef, M.H.G.; Oude Nijeweeme, J.K.; De Bock, G.H.; Berger, M.Y.; Brandenbarg, D. Home-based physical activity to alleviate fatigue in cancer survivors: A systematic review and meta-analysis. Med. Sci. Sport. Exerc. 2021, 53, 2661–2674. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Gillison, F.B.; Skevington, S.M.; Sato, A.; Standage, M.; Evangelidou, S. The effects of exercise interventions on quality of life in clinical and healthy populations: A meta-analysis. Soc. Sci. Med. 2009, 68, 1700–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Tabassum, D.; Baig, S.S.; Moyle, B.; Redgrave, J.; Nichols, S.; McGregor, G.; Evans, K.; Totton, N.; Cooper, C.; et al. Effect of exercise interventions on health-related quality of life after stroke and transient ischemic attack. Stroke 2021, 52, 2445–2455. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Chu, H. The impact of excluding trials from network meta-analyses—An empirical study. PLoS ONE 2016, 11, e0165889. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Coster, S.; Poole, K.; Fallowfield, L.J. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res. Treat. 2001, 68, 273–282. [Google Scholar] [CrossRef]
- Michels, F.A.S.; Latorre, M.R.D.O.; Maciel, M.S. Validation, reliability and comprehension of the IBCSG quality of life questionnaire specific to breast cancer. Appl. Cancer Res. 2010, 30, 348–352. [Google Scholar]
- Groenvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing Effect Measures and Computing Estimates of Effect. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-06 (accessed on 25 February 2023).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 25 February 2023).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 25 February 2023).
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including Variants on Randomized Trials. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-23 (accessed on 25 February 2023).
- Borenstein, M.; Hedges, L.V.; Rothstein, H.R. Fixed-effect versus random-effects models. In Introduction to Meta-Analysis; Borenstein, M., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 77–86. [Google Scholar]
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effect Size. Wikipedia. Available online: https://en.wikipedia.org/wiki/Effect_size (accessed on 17 May 2023).
- Pearson, M.J.; Smart, N.A. Reported methods for handling missing change standard deviations in meta-analyses of exercise therapy interventions in patients with heart failure: A systematic review. PLoS ONE 2018, 13, e0205952. [Google Scholar] [CrossRef] [PubMed]
- Milne, H.M.; Wallman, K.E.; Gordon, S.; Courneya, K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2008, 108, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Baruth, M.; Wilcox, S.; Der Ananian, C.; Heiney, S. Effects of home-based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: A 12-week pilot study. J. Phys. Act. Health 2015, 12, S110–S118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stan, D.L.; Croghan, K.A.; Croghan, I.T.; Jenkins, S.M.; Sutherland, S.J.; Cheville, A.L.; Pruthi, S. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support. Care Cancer 2016, 24, 4005–4015. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, C.; He, C.; Yan, J.; Chen, Y.; Gao, L.; Liu, R.; Cao, B. Effectiveness of three exercise programs and intensive follow-up in improving quality of life, pain, and lymphedema among breast cancer survivors: A randomized, controlled 6-month trial. Support. Care Cancer 2023, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Heiman, J.; Onerup, A.; Bock, D.; Haglind, E.; Olofsson Bagge, R. The effect of nonsupervised physical activity before and after breast cancer surgery on quality of life: Results from a randomized controlled trial (PhysSURG-B). Scand. J. Surg. 2022, 111, 75–82. [Google Scholar] [CrossRef]
- Mostafaei, F.; Azizi, M.; Jalali, A.; Salari, N.; Abbasi, P. Effect of exercise on depression and fatigue in breast cancer women undergoing chemotherapy: A randomized controlled trial. Heliyon 2021, 7, e07657. [Google Scholar] [CrossRef]
- Naczk, A.; Huzarski, T.; Doś, J.; Górska-Doś, M.; Gramza, P.; Gajewska, E.; Naczk, M. Impact of inertial training on muscle strength and quality of life in breast cancer survivors. Int. J. Environ. Res. Public. Health 2022, 19, 3278. [Google Scholar] [CrossRef]
- Schröder, M.L.; Stöckigt, B.; Binting, S.; Tissen-Diabaté, T.; Bangemann, N.; Goerling, U.; Kröz, M.; Blohmer, J.U.; Ortiz, M.; Brinkhaus, B. Feasibility and possible effects of mindful walking and moderate walking in breast cancer survivors: A randomized controlled pilot study with a nested qualitative study part. Integr. Cancer Ther. 2022, 21, 15347354211066067. [Google Scholar] [CrossRef]
- Boing, L.; Fretta, T.B.; Lynch, B.M.; Dias, M.; Rosa, L.M.D.; Baptista, F.; Bergmann, A.; Fausto, D.Y.; Bocchi Martins, J.B.; Guimarães, A.C.A. Mat Pilates and belly dance: Effects on patient-reported outcomes among breast cancer survivors receiving hormone therapy and adherence to exercise. Complement Ther. Clin. Pract. 2023, 50, 101683. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Inbaraj, G.; Sathyaprabha, T.N.; Udupa, K.; Ram, A.; Patil, S.; Rajeswaran, J.; Nandakumar, K.K.; Belur, S.; Singh, A.D.; Prathyusha, P.V.; et al. Impact of integrated yoga therapy on cognitive impairment and cardiac dysfunction in relation to quality of life in breast cancer patients undergoing chemotherapy: Study protocol for a two-arm randomized controlled trial. Front. Oncol. 2022, 12, 955184. [Google Scholar] [CrossRef]
- Ammitzbøll, G.; Kristina Kjær, T.; Johansen, C.; Lanng, C.; Wreford Andersen, E.; Kroman, N.; Zerahn, B.; Hyldegaard, O.; Envold Bidstrup, P.; Oksbjerg Dalton, S. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery—Results from a randomized controlled trial. Acta Oncol. 2019, 58, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gal, R.; Monninkhof, E.M.; van Gils, C.H.; Groenwold, R.H.H.; Elias, S.G.; van den Bongard, D.; Peeters, P.H.M.; Verkooijen, H.M.; May, A.M. Effects of exercise in breast cancer patients: Implications of the trials within cohorts (TwiCs) design in the UMBRELLA Fit trial. Breast Cancer Res. Treat. 2021, 190, 89–101. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, H.J.; Stuiver, M.M.; May, A.M.; van Grinsven, S.; Aaronson, N.K.; Oldenburg, H.S.A.; van der Poel, H.G.; Koole, S.N.; Retèl, V.P.; van Harten, W.H.; et al. Effects of and lessons learned from an internet-based physical activity support program (with and without physiotherapist telephone counselling) on physical activity levels of breast and prostate cancer survivors: The PABLO randomized controlled trial. Cancers 2021, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Mazuquin, B.; Canaway, A.; Hossain, A.; Williamson, E.; Mistry, P.; Lall, R.; Petrou, S.; Lamb, S.E.; Rees, S.; et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): Multicentre randomised controlled trial and economic evaluation. BMJ 2021, 375, e066542. [Google Scholar] [CrossRef] [PubMed]
- Odynets, T.; Briskin, Y.; Todorova, V. Effects of different exercise interventions on quality of life in breast cancer patients: A randomized controlled trial. Integr. Cancer Ther. 2019, 18, 1534735419880598. [Google Scholar] [CrossRef] [Green Version]
- Reeves, M.M.; Terranova, C.O.; Winkler, E.A.H.; McCarthy, N.; Hickman, I.J.; Ware, R.S.; Lawler, S.P.; Eakin, E.G.; Demark-Wahnefried, W. Effect of a remotely delivered weight loss intervention in early-stage breast cancer: Randomized controlled trial. Nutrients 2021, 13, 4091. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Deluche, E.; Bonis, J.; Leobon, S.; Antonini, M.T.; Laval, C.; Favard, F.; Dobbels, E.; Lavau-Denes, S.; Labrunie, A.; et al. Home-based physical activity in patients with breast cancer: During and/or after chemotherapy? Impact on cardiorespiratory fitness. A 3-arm randomized controlled trial (APAC). Integr. Cancer Ther. 2020, 19, 1534735420969818. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Mazuquin, B.; Mistry, P.; Rees, S.; Canaway, A.; Hossain, A.; Williamson, E.; Padfield, E.J.; Lall, R.; Richmond, H.; et al. Exercise to prevent shoulder problems after breast cancer surgery: The PROSPER RCT. Health Technol. Assess. 2022, 26, 1–124. [Google Scholar] [CrossRef]
- Knoerl, R.; Giobbie-Hurder, A.; Sannes, T.S.; Chagpar, A.B.; Dillon, D.; Dominici, L.S.; Frank, E.S.; Golshan, M.; McTiernan, A.; Rhei, E.; et al. Exploring the impact of exercise and mind-body prehabilitation interventions on physical and psychological outcomes in women undergoing breast cancer surgery. Support. Care Cancer 2022, 30, 2027–2036. [Google Scholar] [CrossRef]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Bloomquist, K.; Adamsen, L.; Hayes, S.C.; Lillelund, C.; Andersen, C.; Christensen, K.B.; Oturai, P.; Ejlertsen, B.; Tuxen, M.K.; Møller, T. Heavy-load resistance exercise during chemotherapy in The impact of maximal strength at risk for lymphedema: A randomized trial. Acta Oncol. 2019, 58, 1667–1675. [Google Scholar] [CrossRef]
- Cešeiko, R.; Eglītis, J.; Srebnijs, A.; Timofejevs, M.; Purmalis, E.; Erts, R.; Vētra, A.; Tomsone, S. The impact of maximal strength training on quality of life among women with breast cancer undergoing treatment. Exp. Oncol. 2019, 41, 166–172. [Google Scholar] [CrossRef]
- Jacot, W.; Arnaud, A.; Jarlier, M.; Lefeuvre-Plesse, C.; Dalivoust, P.; Senesse, P.; Azzedine, A.; Tredan, O.; Sadot-Lebouvier, S.; Mas, S.; et al. Brief hospital supervision of exercise and diet during adjuvant breast cancer therapy is not enough to relieve fatigue: A multicenter randomized controlled trial. Nutrients 2020, 12, 3081. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Cormie, P.; Peddle-McIntyre, C.J.; Galvão, D.A.; Taaffe, D.R.; Schofield, C.; Ray, S.; Zissiadis, Y.; Newton, R.U. The effects of home-based exercise therapy for breast cancer-related fatigue induced by radical radiotherapy. Breast Cancer 2023, 30, 139–150. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Hopkins-Price, P.; Vicari, S.; Pamenter, R.; Courneya, K.S.; Markwell, S.; Verhulst, S.; Hoelzer, K.; Naritoku, C.; Jones, L.; et al. A randomized trial to increase physical activity in breast cancer survivors. Med. Sci. Sport. Exerc. 2009, 41, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Wonders, K.Y.; Schmitz, K.; Wise, R.; Hale, R. Cost-savings analysis of an individualized exercise oncology program in early-stage breast cancer survivors: A randomized clinical control trial. JCO Oncol. Pract. 2022, 18, e1170–e1180. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yuan, R.; Yang, J.; Zheng, W.; Jin, Y.; Wang, M.; Jiang, J.; Wu, C.; Li, K. Effects of Baduanjin exercise on cognitive function and cancer-related symptoms in women with breast cancer receiving chemotherapy: A randomized controlled trial. Support. Care Cancer 2022, 30, 6079–6091. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.; Matthews, L.; Visotcky, A.; Banerjee, A.; Moosreiner, A.; Kelley, K.; Chitambar, C.R.; Papanek, P.E.; Stolley, M. Every Day Counts: A randomized pilot lifestyle intervention for women with metastatic breast cancer. Breast Cancer Res. Treat. 2021, 187, 729–741. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Hopkins-Price, P.; Vicari, S.; Markwell, S.; Pamenter, R.; Courneya, K.S.; Hoelzer, K.; Naritoku, C.; Edson, B.; Jones, L.; et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: Persistent and delayed effects. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1410–1418. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.I.; Gonzalez, A.; Moon, C.; Serra, M.; Bridges, P.B.; Hughes, D.; Clarke, G.; Kilpela, L.; Jiwani, R.; Musi, N. Exercise and creatine supplementation to augment the adaptation of exercise training among breast cancer survivors completing chemotherapy: Protocol for an open-label randomized controlled trial (the THRIVE Study). JMIR Res. Protoc. 2022, 11, e26827. [Google Scholar] [CrossRef]
- Smith-Turchyn, J.; McCowan, M.E.; O’Loughlin, E.; Fong, A.J.; McDonough, M.H.; Santa Mina, D.; Arbour-Nicitopoulos, K.P.; Trinh, L.; Jones, J.M.; Bender, J.L.; et al. Connecting breast cancer survivors for exercise: Protocol for a two-arm randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 128. [Google Scholar] [CrossRef]
- Wang, C.C.; Geraghty, S.; Fox-Harding, C.; Wang, C. Effects of a nurse-led Tai Chi programme on improving quality of life, mental wellbeing, and physical function of women with breast cancer: Protocol for a randomized controlled trial. Womens Health 2022, 18, 17455057221127813. [Google Scholar] [CrossRef]
- Lynch, B.M.; Nguyen, N.H.; Reeves, M.M.; Moore, M.M.; Rosenberg, D.E.; Wheeler, M.J.; Boyle, T.; Vallance, J.K.; Friedenreich, C.M.; English, D.R. Study design and methods for the ACTIVity And TEchnology (ACTIVATE) trial. Contemp. Clin. Trials 2018, 64, 112–117. [Google Scholar] [CrossRef]
- Salchow, J.L.; Strunk, M.A.; Niels, T.; Steck, J.; Minto, C.A.; Baumann, F.T. A randomized controlled pilot trial about the influence of Kyusho Jitsu exercise on self-efficacy, fear, depression, and distress of breast cancer patients within follow-up care. Integr. Cancer Ther. 2021, 20, 15347354211037955. [Google Scholar] [CrossRef]
- Lin, H.P.; Kuo, Y.H.; Tai, W.Y.; Liu, H.E. Exercise effects on fatigue in breast cancer survivors after treatments: A systematic review and meta-analysis. Int. J. Nurs. Pract. 2022, 28, e12989. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, J.; Bezerra, E.S.; Winters-Stone, K.M.; Alberto Gobbo, L.; Freitas, I.F.J. Mat Pilates improves lower and upper body strength and flexibility in breast cancer survivors undergoing hormone therapy: A randomized controlled trial (HAPiMat study). Disabil. Rehabil. 2023, 45, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Jacquinot, Q.; Meneveau, N.; Falcoz, A.; Bouhaddi, M.; Roux, P.; Degano, B.; Chatot, M.; Curtit, E.; Mansi, L.; Paillard, M.J.; et al. Cardiotoxicity is mitigated after a supervised exercise program in HER2-positive breast cancer undergoing adjuvant trastuzumab. Front. Cardiovasc. Med. 2022, 9, 1000846. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Courneya, K.S.; Carter, S.J.; Anton, P.M.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; McAuley, E. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res. Treat. 2016, 159, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleicher, E.; McAuley, E.; Courneya, K.S.; Anton, P.; Ehlers, D.K.; Phillips, S.M.; Oster, R.A.; Pekmezi, D.; Rogers, L.Q. Moderators of physical activity and quality of life response to a physical activity intervention for breast cancer survivors. Support. Care Cancer 2022, 31, 53. [Google Scholar] [CrossRef]
- Holtdirk, F.; Mehnert, A.; Weiss, M.; Mayer, J.; Meyer, B.; Bröde, P.; Claus, M.; Watzl, C. Results of the Optimune trial: A randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS ONE 2021, 16, e0251276. [Google Scholar] [CrossRef]
- Duijts, S.F.; van Beurden, M.; Oldenburg, H.S.; Hunter, M.S.; Kieffer, J.M.; Stuiver, M.M.; Gerritsma, M.A.; Menke-Pluymers, M.B.; Plaisier, P.W.; Rijna, H.; et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: Results of a randomized, controlled, multicenter trial. J. Clin. Oncol. 2012, 30, 4124–4133. [Google Scholar] [CrossRef]
- Poier, D.; Büssing, A.; Rodrigues Recchia, D.; Beerenbrock, Y.; Reif, M.; Nikolaou, A.; Zerm, R.; Gutenbrunner, C.; Kröz, M. Influence of a multimodal and multimodal-aerobic therapy concept on health-related quality of life in breast cancer survivors. Integr. Cancer Ther. 2019, 18, 1534735418820447. [Google Scholar] [CrossRef]
- Vallance, J.K.; Nguyen, N.H.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Milton, S.; Friedenreich, C.M.; English, D.R.; Lynch, B.M. Effects of the ACTIVity And TEchnology (ACTIVATE) intervention on health-related quality of life and fatigue outcomes in breast cancer survivors. Psychooncology 2020, 29, 204–211. [Google Scholar] [CrossRef]
- Chan, D.N.S.; Chow, K.M.; Anderson, D.J.; Porter-Steele, J.; Laing, B.; Ling, W.M.; Lam, C.C.H.; Choi, K.C.; Chan, C.W.H.; So, W.K.W.; et al. Cultural adaptation of the younger women’s wellness after cancer program for younger Chinese women with breast cancer: A pilot randomized controlled trial. Cancer Nurs. 2023. [Google Scholar] [CrossRef]
- Naderi, M.; Kordestani, H.; Sahebi, Z.; Khedmati Zare, V.; Amani-Shalamzari, S.; Kaviani, M.; Wiskemann, J.; Molanouri Shamsi, M. Serum and gene expression profile of cytokines following combination of yoga training and vitamin D supplementation in breast cancer survivors: A randomized controlled trial. BMC Womens Health 2022, 22, 90. [Google Scholar] [CrossRef]
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.; Saunders, C.; Zissiadis, Y.; Newton, R.U. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J. Cancer Surviv. 2013, 7, 413–424. [Google Scholar] [CrossRef]
- Buchan, J.; Janda, M.; Box, R.; Schmitz, K.; Hayes, S. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Med. Sci. Sport. Exerc. 2016, 48, 1866–1874. [Google Scholar] [CrossRef]
- Zhi, W.I.; Baser, R.E.; Zhi, L.M.; Talukder, D.; Li, Q.S.; Paul, T.; Patterson, C.; Piulson, L.; Seluzicki, C.; Galantino, M.L.; et al. Yoga for cancer survivors with chemotherapy-induced peripheral neuropathy: Health-related quality of life outcomes. Cancer Med. 2021, 10, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Ax, A.K.; Johansson, B.; Lyth, J.; Nordin, K.; Börjeson, S. Short- and long-term effect of high versus low-to-moderate intensity exercise to optimise health-related quality of life after oncological treatment-results from the Phys-Can project. Support. Care Cancer 2022, 30, 5949–5963. [Google Scholar] [CrossRef]
- Koch, A.K.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G.; Langhorst, J.; Cramer, H. The effects of yoga and self-esteem on menopausal symptoms and quality of life in breast cancer survivors-A secondary analysis of a randomized controlled trial. Maturitas 2017, 105, 95–99. [Google Scholar] [CrossRef]
- McNeil, J.; Fahim, M.; Stone, C.R.; O’Reilly, R.; Courneya, K.S.; Friedenreich, C.M. Adherence to a lower versus higher intensity physical activity intervention in the Breast Cancer & Physical Activity Level (BC-PAL) Trial. J. Cancer Surviv. 2022, 16, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yi, X.; Gao, D.; Gao, Z.; Huang, S.; Chao, M.; Chen, W.; Ding, M. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Strunk, M.A.; Zopf, E.M.; Steck, J.; Hamacher, S.; Hallek, M.; Baumann, F.T. Effects of Kyusho Jitsu on physical activity-levels and quality of life in breast cancer patients. In Vivo 2018, 32, 819–824. [Google Scholar] [CrossRef]
- Patel, S.R.; Zayas, J.; Medina-Inojosa, J.R.; Loprinzi, C.; Cathcart-Rake, E.J.; Bhagra, A.; Olson, J.E.; Couch, F.J.; Ruddy, K.J. Real-World Experiences With Yoga on Cancer-Related Symptoms in Women With Breast Cancer. Glob. Adv. Health Med. 2021, 10, 2164956120984140. [Google Scholar] [CrossRef]
- Danhauer, S.C.; Addington, E.L.; Cohen, L.; Sohl, S.J.; Van Puymbroeck, M.; Albinati, N.K.; Culos-Reed, S.N. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer 2019, 125, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Ostman, C.; Jewiss, D.; Smart, N.A. The effect of exercise training intensity on quality of life in heart failure patients: A systematic review and meta-analysis. Cardiology 2017, 136, 79–89. [Google Scholar] [CrossRef]
- Dysart, A.; Harden, S.M. Effects of temperature and tempo: Evaluating how much time in a typical community-based yoga class is moderate-intensity aerobic activity. Int. J. Environ. Res. Public. Health 2023, 20, 2349. [Google Scholar] [CrossRef] [PubMed]
- Day, M.L.; McGuigan, M.R.; Brice, G.; Foster, C. Monitoring exercise intensity during resistance training using the session RPE scale. J. Strength. Cond. Res. 2004, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bustos, A.; de Pedro, C.G.; Romero-Elías, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Méndez, M.; Fiuza-Luces, C.; Méndez-Otero, M.; et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef]




| First Author & Year | Study Country | Enrolled Population (Age 1) | Participants in Nodes | QoL Scale (Range) | 12-Week QoL Improvement | 12-Week Dropouts | Exercise Details |
|---|---|---|---|---|---|---|---|
| Milne 2008 [43] | Australia | Stage I–II breast cancer completed all treatments except H/T (55.1 ± 8.2) | Aerobic + Strength 29 Control 29 | FACT-B (0–144) | 20.80 ± 7.80 −5.30 ± 8.63 | 0/29 0/29 | The intervention consisted of 20 min of aerobic activity with a 5-min cool down, followed by 12 strength-training movements, each performed for 2 sets of 10–15 repetitions. The intervention was conducted 3 times a week for 12 weeks. |
| Ergun 2013 [6] | Turkey | Stage I–III breast cancer s/p op, C/T, R/T in post-menopause (51.7 ± 8.8) | Aerobic + Strength 20 Aerobic 18 Control 20 | EORTC QLQ-C30 (0–100) | 6.25 ± 11.33 7.73 ± 14.21 −6.67 ± 14.24 | 0/20 2/20 0/20 | Group 1: Strength + Aerobic intervention, which included resistance training for 45 min per day, 3 days per week, and brisk walking for 30 min per day, 3 days per week. Group 2: Aerobic intervention, which included brisk walking for 30 min per day, 3 days per week. Group 3: Control group, which received no specific intervention. |
| Baruth 2015 [44] | USA | Stage I–III breast cancer completed adjuvant treatment in post-menopause (56.5 ± 6.3) | Aerobic 18 Control 12 | IBCSG QOL (0–100) | 5.50 ± 15.81 −3.90 ± 15.42 | 2/20 0/12 | Participants engaged in instructed walking as the intervention, starting from 20 min per day, 3 days per week with an RPE of 3 (on a scale of 0–10), and gradually increasing to 30–40 min per day, 5 days per week with an RPE of 4–6 over the 12-week period. |
| Cramer 2015 [10] | Germany | Stage I–III breast cancer s/p op, C/T, R/T in post-menopause (49.2 ± 5.9) | Yoga 19 Control 21 | FACT-B (0–144) | 10.80 ± 12.87 −1.90 ± 8.89 | 0/19 0/21 | Participants engaged in a Hatha yoga intervention led by a certified instructor for 90 min once per week over a 12-week period. |
| Rogers 2015 [8] | USA | DCIS or Stage I-IIIa breast cancer s/p op, C/T, R/T (54.4 ± 8.5) | Aerobic 106 Control 110 | FACT-B (0–144) | 5.10 ± 11.08 −0.60 ± 12.70 | 4/110 2/112 | The intervention involved gradually increasing aerobic exercise over 12 weeks, starting with 15–20 min, 3 days a week at 40–59% of heart rate reserve and progressing to moderate intensity (>3 times per week, 30–50 min, 40–59% heart rate reserve). |
| Stan 2016 [45] | USA | Stage 0–II breast cancer s/p op, C/T, R/T with cancer-related fatigue (62.1 ± 8.1) | Yoga 14 Strength 9 | FACT-B (0–144) | 5.50 ± 9.70 7.00 ± 10.70 | 4/18 7/16 | Yoga intervention: Participants engaged in a 90-min video program, 3–5 times per week. Strength intervention: Participants engaged in 5 upper and 5 lower body exercises with 8–10 repetitions per exercise, for a total of 20 min, 3–5 times per week. |
| Kim 2020 [11] | Korea | Stage I–III breast cancer completed op and C/T with fatigue (49.2 ± 7.1) | Aerobic + Strength 23 Control 25 | FACT-B (0–144) | 32.85 ± 15.10 28.40 ± 16.10 | 1/24 1/26 | The 12-week comprehensive program included social group interaction and a combination of low-, moderate-, and high-intensity exercises, consisting of both aerobic and strength training. Participants received three exercise sessions per week. |
| Soriano-Maldonado 2022 [9] | Spain | Non-metastatic breast cancer s/p op, C/T, R/T (52.3 ± 9.0) | Strength 32 Control 28 | FACT-B + 4 (0–148) | 0.00 ± 9.62 2.90 ± 9.52 | 0/32 0/28 | Participants engaged in a resistance-training intervention led by an exercise professional, which consisted of 60 min per session, twice per week, for a total of 12 weeks. The resistance training was initiated with a weight load corresponding to 40% of the participants’ 1RM and was gradually increased to 70% of their 1RM weight based on their ability to tolerate the load. |
| Lin 2023 [46] | China | Breast cancer s/p op (51.6 ± 30.7) | Aerobic 145 Aerobic + Strength 47 | FACT-B (0–144) | 7.12 ± 11.72 12.19 ± 12.28 | 5/150 3/50 | Group 0: JME (a 15-min exercise at 60–80% of HRmax, 3 times a day). Group 1: JME with follow-up. Group 2: JME with aerobic activity (30 min, 5 times per week). Groups 0, 1, and 2 were combined as the aerobic exercise intervention. Group 3: JME with resistance training (8 movements with progressive loads, 2–3 times per week). Group 3 was categorized as the aerobic + strength intervention. |
| Aerobic + Strength | 0.18 [−0.90, 1.25] | - | - | 1.42 [0.51, 2.33] |
| 0.48 [−0.40, 1.36] | Aerobic | - | - | 0.71 [−0.18, 1.60] |
| 0.68 [−0.85, 2.21] | 0.200 [−1.32, 1.72] | Yoga | −0.15 [−1.81, 1.51] | 1.16 [−0.42, 2.74] |
| 1.12 [−0.39, 2.62] | 0.64 [−0.86, 2.14] | 0.44 [−0.89, 1.76] | Strength | −0.30 [−1.82, 1.22] |
| 1.31 [0.49, 2.12] | 0.83 [0.03, 1.63] | 0.63 [−0.67, 1.92] | 0.19 [−1.08, 1.46] | Control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-C.; Chen, P.-L.; Liao, W.-C.; Tsai, I.-C. Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Cancers 2023, 15, 3380. https://doi.org/10.3390/cancers15133380
Wang T-C, Chen P-L, Liao W-C, Tsai I-C. Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Cancers. 2023; 15(13):3380. https://doi.org/10.3390/cancers15133380
Chicago/Turabian StyleWang, Tzu-Chieh, Pei-Lun Chen, Wan-Chun Liao, and I-Chen Tsai. 2023. "Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials" Cancers 15, no. 13: 3380. https://doi.org/10.3390/cancers15133380
APA StyleWang, T. -C., Chen, P. -L., Liao, W. -C., & Tsai, I. -C. (2023). Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Cancers, 15(13), 3380. https://doi.org/10.3390/cancers15133380