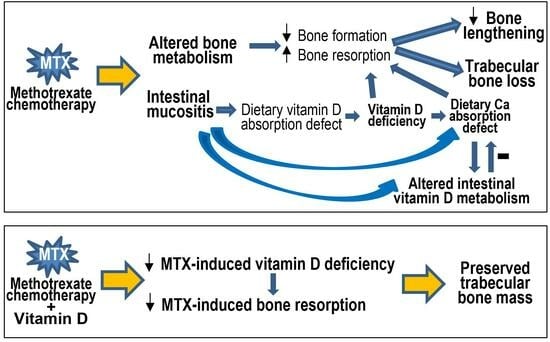
Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.1.1. MTX Treatment Time Course Study
2.1.2. MTX Treatment and Vitamin D Supplementation Trial
2.2. Serum Biochemistry Assays
2.3. Histomorphometric and Micro-Computed Topographical (μ-CT) Analyses of Tibial Trabecular Bone
2.4. RT-PCR Analyses of Vitamin D Metabolism Markers and Ca-Carrier Proteins in Jejunum
2.5. Analyses of MTX-Induced Small Intestine Mucosal Damage and Recovery
2.6. Statistical Analyses
3. Results
3.1. MTX Chemotherapy Caused Body Weight Loss and Decreased Total Body Length Gain and Tibial Length
3.2. MTX Chemotherapy Reduced Serum Vitamin D Levels, Altered Bone Turnover, and Decreased Tibial Trabecular Bone Volume
3.3. MTX Chemotherapy Altered Intestinal Expression of Key Vitamin D Metabolism Enzymes and Ca-Carrier Proteins, which Was Associated with MTX-Induced Intestinal Mucosa Damage
3.4. Vitamin D Supplementation Inhibited MTX Chemotherapy-Induced Bone Loss due to Its Effect in Suppressing Bone Resorption
4. Discussion
4.1. Vitamin D Deficiency, Bone Turnover, Bone Loss, and Ca Homeostasis following MTX Chemotherapy
4.2. Vitamin D Deficiency, Bone Loss, and Altered Intestinal Vitamin D Metabolism Are Linked with MTX-Induced Intestinal Mucositis
4.3. Potential Protective Effect of Vitamin D Supplementation and Action Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaste, S.C.; Jones-Wallace, D.; Rose, S.R.; Boyett, J.M.; Lustig, R.H.; Rivera, G.K.; Pui, C.H.; Hudson, M.M. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia 2001, 15, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C. Skeletal toxicities of treatment in children with cancer. Pediatr. Blood Cancer 2008, 50, 469–473, discussion 486. [Google Scholar] [CrossRef]
- Fan, C.; Foster, B.K.; Wallace, W.H.; Xian, C.J. Pathobiology and prevention of cancer chemotherapy-induced bone growth arrest, bone loss, and osteonecrosis. Curr. Mol. Med. 2011, 11, 140–151. [Google Scholar] [CrossRef]
- te Winkel, M.L.; Pieters, R.; Hop, W.C.; Roos, J.C.; Bokkerink, J.P.; Leeuw, J.A.; Bruin, M.C.; Kollen, W.J.; Veerman, A.J.; de Groot-Kruseman, H.A.; et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the dcog-all9 protocol. Bone 2014, 59, 223–228. [Google Scholar] [CrossRef]
- Su, Y.W.; Chen, K.M.; Hassanshahi, M.; Tang, Q.; Howe, P.R.; Xian, C.J. Childhood cancer chemotherapy-induced bone damage: Pathobiology and protective effects of resveratrol and other nutraceuticals. Ann. N. Y. Acad. Sci. 2017, 1403, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.M.; Rahim, A.; Adams, J.A.; Eden, O.B.; Shalet, S.M. Reduced bone mineral density in young adults following cure of acute lymphoblastic leukaemia in childhood. Br. J. Cancer 1999, 79, 1859–1863. [Google Scholar] [CrossRef]
- Athanassiadou, F.; Tragiannidis, A.; Rousso, I.; Katsos, G.; Sidi, V.; Koliouskas, D.; Papastergiou, C.; Tsituridis, I. Evaluation of bone metabolism in children with acute lymphoblastic leukemia after induction chemotherapy treatment. Pediatr. Hematol. Oncol. 2005, 22, 285–289. [Google Scholar] [CrossRef]
- Davies, J.H.; Evans, B.A.; Jones, E.; Evans, W.D.; Jenney, M.E.; Gregory, J.W. Osteopenia, excess adiposity and hyperleptinaemia during 2 years of treatment for childhood acute lymphoblastic leukaemia without cranial irradiation. Clin. Endocrinol. 2004, 60, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.A.; Halton, J.M.; Bradley, C.; Wu, B.; Barr, R.D. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: Influence of disease, drugs and nutrition. Int. J. Cancer Suppl. 1998, 11, 35–39. [Google Scholar] [CrossRef]
- Ryan, J.W.; Anderson, P.H.; Turner, A.G.; Morris, H.A. Vitamin d activities and metabolic bone disease. Clin. Chim. Acta 2013, 425, 148–152. [Google Scholar] [CrossRef]
- Stallings, V.A. Childhood cancer and vitamins: Prevention and treatment. Pediatr. Blood Cancer 2008, 50, 442–444, discussion 451. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.H.; Chow, E.J.; Koehler, E.; Esbenshade, A.; Smith, L.A.; Sanders, J.; Friedman, D. Significant 25-hydroxyvitamin d deficiency in child and adolescent survivors of acute lymphoblastic leukemia: Treatment with chemotherapy compared with allogeneic stem cell transplant. Pediatr. Blood Cancer 2011, 56, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Revuelta Iniesta, R.; Rush, R.; Paciarotti, I.; Rhatigan, E.B.; Brougham, F.H.M.; McKenzie, J.M.; Wilson, D.C. Systematic review and meta-analysis: Prevalence and possible causes of vitamin d deficiency and insufficiency in pediatric cancer patients. Clin. Nutr. 2016, 35, 95–108. [Google Scholar] [CrossRef]
- Halton, J.M.; Atkinson, S.A.; Fraher, L.; Webber, C.; Gill, G.J.; Dawson, S.; Barr, R.D. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J. Bone Miner. Res. 1996, 11, 1774–1783. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Verma, N.; Kumar, A. Prevalence of vitamin d deficiency in childhood acute lymphoblastic leukemia and its association with adverse outcomes during induction phase of treatment. Nutr. Cancer 2020, 72, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Gahr, K.; Sommers, N.; Bostrom, B. Bone mineral metabolism during chemotherapy in childhood acute lymphoblastic leukemia: Unexpected vitamin d deficiency from induction corticosteroids in acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2021, 43, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Oosterom, N.; Dirks, N.F.; Heil, S.G.; de Jonge, R.; Tissing, W.J.E.; Pieters, R.; van den Heuvel-Eibrink, M.M.; Heijboer, A.C.; Pluijm, S.M.F. A decrease in vitamin d levels is associated with methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. Support. Care Cancer 2019, 27, 183–190. [Google Scholar] [CrossRef]
- van der Sluis, I.M.; van den Heuvel-Eibrink, M.M. Osteoporosis in children with cancer. Pediatr. Blood Cancer 2008, 50, 474–478, discussion 486. [Google Scholar] [CrossRef]
- Huang, T.H.; Liu, H.C.; Hou, J.Y.; Chang, C.Y.; Sun, F.J.; Yeh, T.C. Efficacy and safety of denosumab therapy for low bone mineral density in childhood cancer survivors: A report of preliminary experience. Pediatr. Blood Cancer 2019, 66, e27927. [Google Scholar] [CrossRef]
- Cohen, J.E.; Wakefield, C.E.; Cohn, R.J. Nutritional interventions for survivors of childhood cancer. Cochrane Database Syst. Rev. 2016, 2016, CD009678. [Google Scholar] [CrossRef]
- van Atteveld, J.E.; Verhagen, I.E.; van den Heuvel-Eibrink, M.M.; van Santen, H.M.; van der Sluis, I.M.; Di Iorgi, N.; Simmons, J.H.; Ward, L.M.; Neggers, S. Vitamin d supplementation for children with cancer: A systematic review and consensus recommendations. Cancer Med. 2021, 10, 4177–4194. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Vitamin d: A critical regulator of intestinal physiology. JBMR Plus 2021, 5, e10554. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; Scherer, M.A.; Macsai, C.E.; Fan, C.; Covino, M.; Foster, B.K. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone 2007, 41, 842–850. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone: Short-term methotrexate and dororubicin treatment in a rat model. J. Bone Joint Surg. 1984, 66, 602–607. [Google Scholar] [CrossRef]
- Xian, C.J.; Howarth, G.S.; Mardell, C.E.; Cool, J.C.; Familari, M.; Read, L.C.; Giraud, A.S. Temporal changes in tff3 expression and jejunal morphology during methotrexate-induced damage and repair. Am. J. Physiol. 1999, 277, G785–G795. [Google Scholar] [CrossRef]
- Henley, C.; Colloton, M.; Cattley, R.C.; Shatzen, E.; Towler, D.A.; Lacey, D.; Martin, D. 1,25-dihydroxyvitamin d3 but not cinacalcet hcl (sensipar/mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2005, 20, 1370–1377. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, S.D. Relationship between serum 25-hydroxyvitamin d and lower extremity arterial disease in type 2 diabetes mellitus patients and the analysis of the intervention of vitamin d. J. Diabetes Res. 2015, 2015, 815949. [Google Scholar] [CrossRef] [PubMed]
- Barratt, K.R.; Sawyer, R.K.; Atkins, G.J.; St-Arnaud, R.; Anderson, P.H. Vitamin d supplementation improves bone mineralisation independent of dietary phosphate in male x-linked hypophosphatemic (hyp) mice. Bone 2021, 143, 115767. [Google Scholar] [CrossRef]
- Fan, C.; Georgiou, K.R.; McKinnon, R.A.; Keefe, D.M.; Howe, P.R.; Xian, C.J. Combination chemotherapy with cyclophosphamide, epirubicin and 5-fluorouracil causes trabecular bone loss, bone marrow cell depletion and marrow adiposity in female rats. J. Bone Miner. Metab. 2015, 34, 277–290. [Google Scholar] [CrossRef]
- Lee, A.M.; Shandala, T.; Nguyen, L.; Muhlhausler, B.S.; Chen, K.; Howe, P.R.; Xian, C.J. Effects of resveratrol supplementation on bone growth in young rats and microarchitecture and remodeling in ageing rats. Nutrients 2014, 6, 5871–5887. [Google Scholar] [CrossRef]
- Su, Y.W.; Wong, D.S.K.; Fan, J.; Chung, R.; Wang, L.; Chen, Y.; Xian, C.H.; Yao, L.; Wang, L.; Foster, B.K.; et al. Enhanced bmp signalling causes growth plate cartilage dysrepair in rats. Bone 2021, 145, 115874. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; O’Loughlin, P.D.; May, B.K.; Morris, H.A. Quantification of mrna for the vitamin d metabolizing enzymes cyp27b1 and cyp24 and vitamin d receptor in kidney using real-time reverse transcriptase- polymerase chain reaction. J. Mol. Endocrinol. 2003, 31, 123–132. [Google Scholar] [CrossRef]
- Charoenphandhu, N.; Teerapornpuntakit, J.; Lapmanee, S.; Krishnamra, N.; Charoenphandhu, J. Duodenal calcium transporter mrna expression in stressed male rats treated with diazepam, fluoxetine, reboxetine, or venlafaxine. Mol. Cell Biochem. 2012, 369, 87–94. [Google Scholar] [CrossRef]
- Howarth, G.; Francis, G.; Cool, J.; Xu, X.; Byard, R.; Read, L. Milk growth factor enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J. Nutr. 1996, 126, 2519–2530. [Google Scholar] [CrossRef]
- Xian, C.J.; Cool, J.C.; Howarth, G.S.; Read, L.C. Effects of tgf-alpha gene knockout on epithelial cell kinetics and repair of methotrexate-induced damage in mouse small intestine. J. Cell Physiol. 2002, 191, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Cool, J.; Xian, C.J. Dietary zinc and metallothionein on small intestinal disaccharidases activity in mice. World J. Gastroenterol. 2011, 17, 354–360. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin d deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Khattar, V.; Wang, L.; Peng, J.B. Calcium selective channel trpv6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef]
- Aita, R.; Aldea, D.; Hassan, S.; Hur, J.; Pellon-Cardenas, O.; Cohen, E.; Chen, L.; Shroyer, N.; Christakos, S.; Verzi, M.P.; et al. Genomic analysis of 1,25-dihydroxyvitamin d(3) action in mouse intestine reveals compartment and segment-specific gene regulatory effects. J. Biol. Chem. 2022, 298, 102213. [Google Scholar] [CrossRef]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin d3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef]
- Hasan, M.; Oster, M.; Reyer, H.; Ponsuksili, S.; Murani, E.; Wolf, P.; Fischer, D.C.; Wimmers, K. Tissue-wide expression of genes related to vitamin d metabolism and fgf23 signaling following variable phosphorus intake in pigs. Metabolites 2022, 12, 729. [Google Scholar] [CrossRef]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of vitamin d3 by cytochromes p450. Front. Biosci. 2005, 10, 119–134. [Google Scholar] [PubMed]
- Sim, J.Y.; Jung, E.M.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Transcriptional and translational expression of calbindin-d9k in the duodenum, kidney and uterus of a female canine model. J. Vet. Sci. 2010, 11, 15–19. [Google Scholar] [CrossRef]
- Schundeln, M.M.; Hauffa, P.K.; Bauer, J.J.; Temming, P.; Sauerwein, W.; Biewald, E.; Bornfeld, N.; Hauffa, B.P.; Grasemann, C. Pediatric survivors of retinoblastoma are at risk for altered bone metabolism after chemotherapy treatment early in life. Pediatr. Hematol. Oncol. 2015, 32, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin d physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Andrews, C.; McMahon, J.; Muindi, J.R. A prospective clinical trial of cholecalciferol 2000 iu/day in colorectal cancer patients: Evidence of a chemotherapy-response interaction. Anticancer Res. 2012, 32, 1333–1338. [Google Scholar]
- Charehbili, A.; Hamdy, N.A.; Smit, V.T.; Kessels, L.; van Bochove, A.; van Laarhoven, H.W.; Putter, H.; Meershoek-Klein Kranenbarg, E.; van Leeuwen-Stok, A.E.; van der Hoeven, J.J.; et al. Vitamin d (25-0h d3) status and pathological response to neoadjuvant chemotherapy in stage ii/iii breast cancer: Data from the neozotac trial (boog 10-01). Breast 2016, 25, 69–74. [Google Scholar] [CrossRef]
- Kaste, S.C.; Qi, A.; Smith, K.; Surprise, H.; Lovorn, E.; Boyett, J.; Ferry, R.J., Jr.; Relling, M.V.; Shurtleff, S.A.; Pui, C.H.; et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (all). Pediatr. Blood Cancer 2014, 61, 885–893. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Kapoor, G.; Virmani, A.; Bajpai, R. No impact of disease and its treatment on bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2017, 64, e26271. [Google Scholar] [CrossRef]
- Frisk, P.; Arvidson, J.; Ljunggren, O.; Gustafsson, J. Decreased bone mineral density in young adults treated with sct in childhood: The role of 25-hydroxyvitamin d. Bone Marrow Transplant. 2012, 47, 657–662. [Google Scholar] [CrossRef] [PubMed]
- King, T.J.; Georgiou, K.R.; Cool, J.C.; Scherer, M.A.; Ang, E.S.; Foster, B.K.; Xu, J.; Xian, C.J. Methotrexate chemotherapy promotes osteoclast formation in the long bone of rats via increased pro-inflammatory cytokines and enhanced nf-κb activation. Am. J. Pathol. 2012, 181, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Quattrini, S.; Palpacelli, A.; Catassi, G.N.; Lionetti, M.E.; Catassi, C. Metabolic bone disease in children with intestinal failure and long-term parenteral nutrition: A systematic review. Nutrients 2022, 14, 995. [Google Scholar] [CrossRef]
- Anderson, P.H. Vitamin d activity and metabolism in bone. Curr. Osteoporos. Rep. 2017, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Janousek, J.; Pilarova, V.; Macakova, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remiao, F.; Saso, L.; Mala-Ladova, K.; Maly, J.; et al. Vitamin d: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin d and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Chanpaisaeng, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal calcium absorption. Compr. Physiol. 2021, 11, 2047–2073. [Google Scholar]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin d and phosphate interactions in health and disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar]
- Torremade, N.; Bozic, M.; Goltzman, D.; Fernandez, E.; Valdivielso, J.M. Effects of the administration of 25(oh) vitamin d3 in an experimental model of chronic kidney disease in animals null for 1-alpha-hydroxylase. PLoS ONE 2017, 12, e0170654. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin d: Production, metabolism and mechanisms of action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Zhang, S.; Miller, D.D.; Li, W. Non-musculoskeletal benefits of vitamin d beyond the musculoskeletal system. Int. J. Mol. Sci. 2021, 22, 2128. [Google Scholar] [CrossRef]
- Gawlik, A.; Gepstein, V.; Rozen, N.; Dahan, A.; Ben-Yosef, D.; Wildbaum, G.; Verbitsky, O.; Shaoul, R.; Weisman, Y.; Tiosano, D. Duodenal expression of 25 hydroxyvitamin d3-1alpha-hydroxylase is higher in adolescents than in children and adults. J. Clin. Endocrinol. Metab. 2015, 100, 3668–3675. [Google Scholar] [CrossRef]
- Allen, A.; Bell, A.; Mantle, M.; Pearson, J.P. The structure and physiology of gastrointestinal mucus. Adv. Exp. Med. Biol. 1982, 144, 115–133. [Google Scholar] [PubMed]
- Xian, C.J.; Couper, R.; Howarth, G.S.; Read, L.C.; Kallincos, N.C. Increased expression of hgf and c-met in rat small intestine during recovery from methotrexate-induced mucositis. Br. J. Cancer 2000, 82, 945–952. [Google Scholar] [CrossRef]
- Kolli, V.K.; Natarajan, K.; Isaac, B.; Selvakumar, D.; Abraham, P. Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum. Exp. Toxicol. 2014, 33, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Jahovic, N.; Sener, G.; Cevik, H.; Ersoy, Y.; Arbak, S.; Yegen, B.C. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem. Funct. 2004, 22, 169–178. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Mithal, A.; Gupta, S.; Shukla, M.; Chadha, A. Effect of vitamin d supplementation on bone turnover markers in children and adolescents from north india. Indian. J. Endocrinol. Metab. 2019, 23, 27–34. [Google Scholar] [CrossRef]
- Nahas-Neto, J.; Cangussu, L.M.; Orsatti, C.L.; Bueloni-Dias, F.N.; Poloni, P.F.; Schmitt, E.B.; Nahas, E.A.P. Effect of isolated vitamin d supplementation on bone turnover markers in younger postmenopausal women: A randomized, double-blind, placebo-controlled trial. Osteoporos. Int. 2018, 29, 1125–1133. [Google Scholar] [CrossRef]
- Brandi, M.L. Indications on the use of vitamin d and vitamin d metabolites in clinical phenotypes. Clin. Cases Miner. Bone Metab. 2010, 7, 243–250. [Google Scholar] [PubMed]
- Lung, B.E.; Mowery, M.L.; Komatsu, D.E.E. Calcitriol. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pike, J.W.; Christakos, S. Biology and mechanisms of action of the vitamin d hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin d and the host-gut microbiome: A brief overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Sylvester, C.L.; Anderson, P.H.; Stringer, A.M. New therapeutic strategies for combatting gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2020, 14, 142–152. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rybakovsky, E.; Abdavies, R.; Chamoun, R.; Flounders, C.A.; Shepley-McTaggart, A.; Harty, R.N.; Mullin, J.M. Micronutrient improvement of epithelial barrier function in various disease states: A case for adjuvant therapy. Int. J. Mol. Sci. 2022, 23, 2995. [Google Scholar] [CrossRef]
- Zhou, A.; Hypponen, E. Vitamin d deficiency and c-reactive protein: A bidirectional mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef]
- Wei, L.; Wen, X.S.; Xian, C.J. Chemotherapy-induced intestinal microbiota dysbiosis impairs mucosal homeostasis by modulating toll-like receptor signaling pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin d regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-W.; Lee, A.M.C.; Xu, X.; Hua, B.; Tapp, H.; Wen, X.-S.; Xian, C.J. Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation. Cancers 2023, 15, 4367. https://doi.org/10.3390/cancers15174367
Su Y-W, Lee AMC, Xu X, Hua B, Tapp H, Wen X-S, Xian CJ. Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation. Cancers. 2023; 15(17):4367. https://doi.org/10.3390/cancers15174367
Chicago/Turabian StyleSu, Yu-Wen, Alice M. C. Lee, Xukang Xu, Belinda Hua, Heather Tapp, Xue-Sen Wen, and Cory J. Xian. 2023. "Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation" Cancers 15, no. 17: 4367. https://doi.org/10.3390/cancers15174367
APA StyleSu, Y. -W., Lee, A. M. C., Xu, X., Hua, B., Tapp, H., Wen, X. -S., & Xian, C. J. (2023). Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation. Cancers, 15(17), 4367. https://doi.org/10.3390/cancers15174367