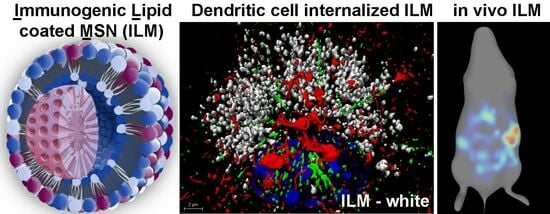
Specific Tumor Localization of Immunogenic Lipid-Coated Mesoporous Silica Nanoparticles following Intraperitoneal Administration in a Mouse Model of Serous Epithelial Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of ~7 nm Dendritic Pores Monodisperse MSNs: Bare (MSN) or Carboxylic Acid-Terminated (MSN-COOH) or Primary Amine-Terminated (MSN-NH2)
2.3. Conjugation of Fluorescent Labels on MSN-NH2
2.4. Preparation of Immunogenic Liposomes
2.5. Ovalbumin Loading Procedure and ILM Assembly
2.6. OVA Loading Quantitation Assay
2.7. Phosphotungstic Acid-Based Negative Staining of ILM for TEM Imaging
2.8. ILM Physical Characterization
2.9. Cell Culture
2.10. Flow Cytometry Analysis of Particle Association with Cells and Surface Markers
2.11. Confocal Microscopy Imaging of Nanoparticle Cellular Association
2.12. ILM Biodistribution Studies
2.13. Tissue Immunofluorescence
2.14. Statistical Analysis
3. Results
3.1. MSN Design and Characterization
3.2. Optimization of Lipid Composition
3.3. DC Activation and Antigen Processing by ILM Formulation
3.4. Cell Type-Dependent Internalization of Anionic ILM
3.5. Lysosomal Tubulation and Streaming in DC following ILM Activation
3.6. ILM Biodistribution in a Mouse Model of Disseminated Ovarian Cancer
3.7. In Vivo Cellular Uptake of ILM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radha, G.; Lopus, M. The spontaneous remission of cancer: Current insights and therapeutic significance. Trans. Oncol. 2021, 14, 101166. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Beyaert, R. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, F.; Wei, F.; Ren, X. The role of toll-like receptor 4 in tumor microenvironment. Oncotarget 2017, 8, 66656–66667. [Google Scholar] [CrossRef]
- MacLeod, M.K.L.; McKee, A.S.; David, A.; Wang, J.; Mason, R.; Kappler, J.W.; Marrack, P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7914–7919. [Google Scholar] [CrossRef]
- Tefit, J.N.; Serra, V. Outlining novel cellular adjuvant products for therapeutic vaccines against cancer. Expert Rev. Vaccines 2011, 10, 1207–1220. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef]
- Marigo, I.; Bosio, E.; Solito, S.; Mesa, C.; Fernandez, A.; Dolcetti, L.; Ugel, S.; Sonda, N.; Bicciato, S.; Falisi, E.; et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ Transcription Factor. Immunity 2010, 32, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, U.K.; Conejo-Garcia, J.R. Modulating the tumor microenvironment as an ovarian cancer treatment strategy. Expert Rev. Obstet. Gynecol. 2012, 7, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, A.; Maestas-Olguin, A.; Saada, E.A.; LaBauve, A.E.; Agola, J.O.; Baty, K.E.; Howard, T.; Sabo, J.K.; Espinoza, C.R.S.; Doudna, J.A.; et al. Engineering of monosized lipid-coated mesoporous silica nanoparticles for CRISPR delivery. Acta Biomater. 2020, 114, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; De May, H.; Franco, S.; Noureddine, A.; Tang, L.; Brinker, C.J.; Kusewitt, D.F.; Adams, S.F.; Serda, R.E. Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nat. Biomed. Eng. 2022, 6, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saric, A.; Hipolito, V.E.B.; Kay, J.G.; Canton, J.; Antonescu, C.N.; Botelho, R.J. mTOR controls lysosome tubulation and antigen presentation in macrophages and dendritic cells. Mol. Biol. Cell 2016, 27, 321–333. [Google Scholar] [CrossRef]
- Mrakovic, A.; Kay, J.G.; Furuya, W.; Brumell, J.H.; Botelho, R.J. Rab7 and Arl8 GTPases are necessary for lysosome tubulation in macrophages. Traffic 2012, 13, 1667–1679. [Google Scholar] [CrossRef]
- ECompeer, E.B.; Flinsenberg, T.W.; Boon, L.; Hoekstra, M.E.; Boes, M. Tubulation of endosomal structures in human dendritic cells by Toll-like receptor ligation and lymphocyte contact accompanies antigen cross-presentation. J. Biol. Chem. 2014, 289, 520–528. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Zajac, A.L.; Twelvetrees, A.; Holzbaur, E.L.F.; Amigorena, S.; Marks, M.S. TLR-dependent phagosome tubulation in dendritic cells promotes phagosome cross-talk to optimize MHC-II antigen presentation. Proc. Natl. Acad. Sci. USA 2014, 111, 15508–15513. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 2013, 14, 135–152. [Google Scholar] [CrossRef]
- Serda, R.E.; Blanco, E.; Mack, A.; Stafford, S.J.; Amra, S.; Li, Q.; van de Ven, A.; Tanaka, T.; Torchilin, V.P.; Wiktorowicz, J.E.; et al. Proteomic analysis of serum opsonins impacting biodistribution and cellular association of porous silicon microparticles. Mol. Imaging 2011, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.-S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 4551. [Google Scholar] [CrossRef] [PubMed]
- Haber, T.; Cornejo, Y.R.; Aramburo, S.; Flores, L.; Cao, P.; Liu, A.; Mooney, R.; Gilchrist, M.; Tirughana, R.; Nwokafor, U.; et al. Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 19737–19745. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, E.W.; Gerber, S.A.; Sedlacek, A.L.; Rybalko, V.Y.; Chan, W.M.; Lord, E.M. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol. Res. 2009, 45, 185–194. [Google Scholar] [CrossRef]
- Oosterling, S.J.; van der Bij, G.J.; Bögels, M.; van der Sijp, J.R.M.; Beelen, R.H.J.; Meijer, S.; van Egmond, M. Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol. Immunother. 2006, 55, 1043–1051. [Google Scholar] [CrossRef]
- Kasagi, Y.; Harada, Y.; Morodomi, Y.; Iwai, T.; Saito, S.; Yoshida, K.; Oki, E.; Saeki, H.; Ohgaki, K.; Sugiyama, M.; et al. Peritoneal Dissemination Requires an Sp1-Dependent CXCR4/CXCL12 Signaling Axis and Extracellular Matrix-Directed Spheroid Formation. Cancer Res. 2016, 76, 347–357. [Google Scholar] [CrossRef]
- Cruz-Migoni, S.; Caamaño, J. Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Front. Immunol. 2016, 7, 612. [Google Scholar] [CrossRef]
- Li, Z.; Fang, X.; Wang, S. Omentum provides a special cell microenvironment for ovarian cancer. Cancer Rep. 2023, e1858. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bilecz, A.J.; Lengyel, E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022, 41, 575–587. [Google Scholar] [CrossRef]
- Heine, H.; Zamyatina, A. Therapeutic Targeting of TLR4 for Inflammation, Infection, and Cancer: A Perspective for Disaccharide Lipid A Mimetics. Pharmaceuticals 2022, 16, 23. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noureddine, A.; Marwedel, B.; Tang, L.; Medina, L.Y.; Serda, R.E. Specific Tumor Localization of Immunogenic Lipid-Coated Mesoporous Silica Nanoparticles following Intraperitoneal Administration in a Mouse Model of Serous Epithelial Ovarian Cancer. Cancers 2023, 15, 4626. https://doi.org/10.3390/cancers15184626
Noureddine A, Marwedel B, Tang L, Medina LY, Serda RE. Specific Tumor Localization of Immunogenic Lipid-Coated Mesoporous Silica Nanoparticles following Intraperitoneal Administration in a Mouse Model of Serous Epithelial Ovarian Cancer. Cancers. 2023; 15(18):4626. https://doi.org/10.3390/cancers15184626
Chicago/Turabian StyleNoureddine, Achraf, Benjamin Marwedel, Lien Tang, Lorel Y. Medina, and Rita E. Serda. 2023. "Specific Tumor Localization of Immunogenic Lipid-Coated Mesoporous Silica Nanoparticles following Intraperitoneal Administration in a Mouse Model of Serous Epithelial Ovarian Cancer" Cancers 15, no. 18: 4626. https://doi.org/10.3390/cancers15184626
APA StyleNoureddine, A., Marwedel, B., Tang, L., Medina, L. Y., & Serda, R. E. (2023). Specific Tumor Localization of Immunogenic Lipid-Coated Mesoporous Silica Nanoparticles following Intraperitoneal Administration in a Mouse Model of Serous Epithelial Ovarian Cancer. Cancers, 15(18), 4626. https://doi.org/10.3390/cancers15184626