Patient-Reported Outcomes among Multiple Myeloma Patients Treated with Standard of Care Idecabtagene Vicleucel
Abstract
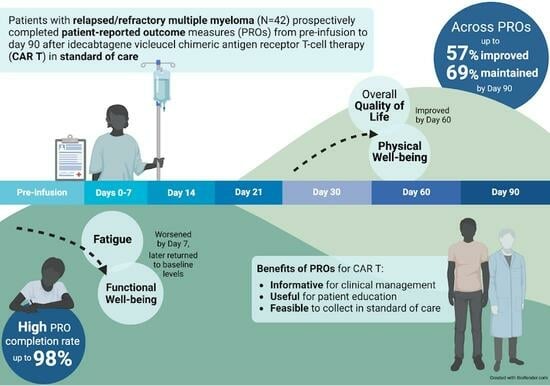
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Participants and Procedures
2.2. Measures
2.2.1. Participant Characteristics and Clinical Outcomes
2.2.2. HRQOL
2.2.3. Symptom Burden
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Baseline PROs
3.3. Mean PRO Changes from Baseline
3.4. Proportions with Clinically Meaningful PRO Scores
3.5. Time to Stable PRO Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; van de Donk, N.; Zweegman, S.; Lokhorst, H.M. Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update. Drugs 2018, 78, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Rifkin, R.M.; Gasparetto, C.J.; Toomey, K.; Durie, B.G.M.; Hardin, J.W.; Terebelo, H.R.; Wagner, L.; Narang, M.; Ailawadhi, S.; et al. Treatment Journeys of Patients With Newly Diagnosed Multiple Myeloma (NDMM): Results From The Connect MM Registry. Clin. Lymphoma Myeloma Leuk. 2020, 20, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406. [Google Scholar] [CrossRef]
- Baz, R.; Lin, H.M.; Hui, A.M.; Harvey, R.D.; Colson, K.; Gallop, K.; Swinburn, P.; Laubach, J.; Berg, D.; Richardson, P. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support. Care Cancer 2015, 23, 2789–2797. [Google Scholar] [CrossRef]
- Johnsen, A.T.; Tholstrup, D.; Petersen, M.A.; Pedersen, L.; Groenvold, M. Health related quality of life in a nationally representative sample of haematological patients. Eur. J. Haematol. 2009, 83, 139–148. [Google Scholar] [CrossRef]
- Boland, E.; Eiser, C.; Ezaydi, Y.; Greenfield, D.M.; Ahmedzai, S.H.; Snowden, J.A. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J. Pain. Symptom Manag. 2013, 46, 671–680. [Google Scholar] [CrossRef]
- Jordan, K.; Proskorovsky, I.; Lewis, P.; Ishak, J.; Payne, K.; Lordan, N.; Kyriakou, C.; Williams, C.D.; Peters, S.; Davies, F.E. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Support. Care Cancer 2014, 22, 417–426. [Google Scholar] [CrossRef]
- Robinson Jr, D.; Esseltine, D.L.; Regnault, A.; Meunier, J.; Liu, K.; van de Velde, H. The influence of baseline characteristics and disease stage on health-related quality of life in multiple myeloma: Findings from six randomized controlled trials. Br. J. Haematol. 2016, 174, 368–381. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jarden, M.; Andersen, C.L.; Frederiksen, H.; Abildgaard, N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur. J. Haematol. 2017, 99, 3–17. [Google Scholar] [CrossRef]
- ABECMA (Idecabtagene Vicleucel) [Package Insert]; Celgene; Bristol-Myers Squibb Company; Bluebird Bio: Summit, NJ, USA, 2021.
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Jagannath, S.; Lin, Y.; Goldschmidt, H.; Reece, D.; Nooka, A.; Senin, A.; Rodriguez-Otero, P.; Powles, R.; Matsue, K.; Shah, N. KarMMa-RW: Comparison of idecabtagene vicleucel with real-world outcomes in relapsed and refractory multiple myeloma. Blood Cancer J. 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Shah, N.; Miguel, J.S.F.; Braverman, J.; Dhanda, D.S.; Shi, L.; Guo, S.; Yu, P.; Liao, W.; Campbell, T.B. Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv. 2022, 6, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Patel, K.K.; Eliason, L.; Dhanda, D.; Shi, L.; Guo, S.; Marshall, T.; Arnulf, B.; Cavo, M.; Nooka, A.K. Health related quality of life (HRQoL) in patients with triple-class-exposed relapsed/refractory multiple myeloma (TCE RRMM) treated with idecabtagene vicleucel (ide-cel) versus standard regimens: Patient-reported outcomes (PROs) from KarMMa-3 phase 3 randomized controlled trial (RCT). J. Clin. Oncol. 2023, 41, 8032. [Google Scholar]
- Booth, C.M.; Tannock, I.F. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.P.; Harrison, M.R.; Walker, M.S.; George, D.J.; Abernethy, A.P.; Hirsch, B.R. Clinical Trial Participants With Metastatic Renal Cell Carcinoma Differ From Patients Treated in Real-World Practice. J. Oncol. Prac. 2015, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: Real-world experience from the myeloma CAR T consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sidana, S.; Shah, G.L.; Scordo, M.; Hamilton, B.K.; Majhail, N.S. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol. Blood Marrow Transpl. 2019, 25, e155–e162. [Google Scholar] [CrossRef]
- Oswald, L.B.; Li, X.; Carvajal, R.; Hoogland, A.I.; Gudenkauf, L.M.; Hansen, D.K.; Alsina, M.; Locke, F.L.; Rodriguez, Y.; Irizarry-Arroyo, N.; et al. Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization. Cancers 2022, 14, 2742. [Google Scholar] [CrossRef]
- Patridge, E.F.; Bardyn, T.P. Research electronic data capture (REDCap). J. Med. Libr. Assoc. JMLA 2018, 106, 142. [Google Scholar] [CrossRef]
- Katz, J.N.; Chang, L.C.; Sangha, O.; Fossel, A.H.; Bates, D.W. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care 1996, 34, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 1 March 2021).
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Pearman, T.; Yanez, B.; Peipert, J.; Wortman, K.; Beaumont, J.; Cella, D. Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer 2014, 120, 2902–2909. [Google Scholar] [CrossRef]
- King, M.T.; Cella, D.; Osoba, D.; Stockler, M.; Eton, D.; Thompson, J.; Eisenstein, A. Meta-analysis provides evidence-based interpretation guidelines for the clinical significance of mean differences for the FACT-G, a cancer-specific quality of life questionnaire. Patient Relat. Outcome Meas. 2010, 1, 119–126. [Google Scholar] [CrossRef]
- Cella, D.; Hahn, E.A.; Dineen, K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual. Life Res. 2002, 11, 207–221. [Google Scholar] [CrossRef]
- Yanez, B.; Pearman, T.; Lis, C.G.; Beaumont, J.L.; Cella, D. The FACT-G7: A rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med. Care 2007, 45 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Spritzer, K.L.; Schalet, B.D.; Cella, D. PROMISA (R)-29 v2.0 profile physical and mental health summary scores. Qual. Life Res. 2018, 27, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Peipert, J.D.; Chapman, R.; Lai, J.-S.; Terluin, B.; Cella, D.; Griffith, P.; Mokkink, L.B. Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual. Life Res. 2021, 30, 2729–2754. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Eton, D.T.; Garcia, S.F.; Cella, D. Minimally important differences were estimated for six PROMIS-Cancer scales in advanced-stage cancer patients. J. Clin. Epidemiol. 2011, 64, 507. [Google Scholar] [CrossRef]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Basch, E.; Becker, C.; Rogak, L.J.; Schrag, D.; Reeve, B.B.; Spears, P.; Smith, M.L.; Gounder, M.M.; Mahoney, M.R.; Schwartz, G.K.; et al. Composite grading algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Clin. Trials 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Gresham, G.; Diniz, M.A.; Razaee, Z.S.; Luu, M.; Kim, S.; Hays, R.D.; Piantadosi, S.; Tighiouart, M.; Yothers, G.; Ganz, P.A.; et al. Evaluating Treatment Tolerability in Cancer Clinical Trials Using the Toxicity Index. J. Natl. Cancer Inst. 2020, 112, 1266–1274. [Google Scholar] [CrossRef]
- Rogatko, A.; Babb, J.S.; Wang, H.; Slifker, M.J.; Hudes, G.R. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin. Cancer Res. 2004, 10, 4645–4651. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar]
- Niscola, P.; Scaramucci, L.; Efficace, F. Towards the Integration of Patient-Reported Outcomes into the Global Clinical Management of Multiple Myeloma. Expert Rev. Hematology. 2019, 12, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.P.; Ganz, P.A.; Murphy, S.B.; Nass, S.J.; Ferrell, B.R.; Stovall, E. Patient-Centered Cancer Treatment Planning: Improving the Quality of Oncology Care. Summary of an Institute of Medicine Workshop. Oncologist 2011, 16, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.C.; Studts, J.L.; Hayslip, J.W. Shared decision making in oncology practice: What do oncologists need to know? Oncologist 2012, 17, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Shahzad, M.; Shippey, E.; Bansal, R.; Mushtaq, M.U.; Mahmoudjafari, Z.; Faisal, M.S.; Hoffmann, M.; Abdallah, A.O.; Divine, C.; et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transpl. Cell Ther. 2022, 28, 358–364. [Google Scholar] [CrossRef]
- Emole, J.; Lawal, O.; Lupak, O.; Dias, A.; Shune, L.; Yusuf, K. Demographic differences among patients treated with chimeric antigen receptor T-cell therapy in the United States. Cancer Med. 2022, 11, 4440–4448. [Google Scholar] [CrossRef]







| Characteristics | n (%) |
|---|---|
| Median age, years (range) | 66 (43–81) |
| Sex | |
| Female | 21 (50) |
| Male | 21 (50) |
| Race/ethnicity | |
| Non-Hispanic White | 29 (69) |
| Hispanic | 6 (14) |
| Non-Hispanic Black | 5 (12) |
| Non-Hispanic American Indian or Alaska Native | 1 (2) |
| Non-Hispanic Asian | 1 (2) |
| Married | 29 (69) |
| Employment | |
| Retired | 23 (55) |
| Employed full-time or part-time | 9 (21) |
| Disabled or on leave without pay | 9 (21) |
| Homemaker | 1 (2) |
| Highest education completed | |
| High school | 4 (10) |
| Partial college or specialized training | 14 (33) |
| College or university | 14 (33) |
| Graduate degree | 10 (24) |
| Annual household income | |
| <USD 60,000 | 17 (41) |
| USD 60,000–USD 100,000 | 13 (31) |
| >USD 100,000 | 7 (17) |
| Prefer not to report | 5 (12) |
| Median Charlson Comorbidity Index (range) | 2 (2–18) |
| Extramedullary disease | 17 (41) |
| High marrow burden | 11 (26) |
| ECOG performance status 0–1 | |
| 0–1 | 38 (91) |
| ≥2 | 4 (10) |
| R-ISS stage | |
| I | 7 (17) |
| II | 29 (69) |
| III | 4 (10) |
| Unknown | 2 (5) |
| Myeloma subtype | |
| Intact immunoglobin | 25 (60) |
| Light chain | 14 (33) |
| Non-secretory | 3 (7) |
| High-risk cytogenetic abnormalities | |
| Any | 15 (36) |
| del(17p) | 11 (26) |
| t(4;14) | 2 (5) |
| t(14;16) | 3 (7) |
| Bridging therapy | 22 (52) |
| Prior therapies * | |
| Median prior therapies (range) | 6 (4–16) |
| Prior autologous stem cell transplant | 36 (86) |
| Prior anti-BCMA therapy | 7 (21) |
| Refractory status * | |
| Refractory to immunomodulatory agents | 39 (93) |
| Refractory to proteasome inhibitors | 37 (88) |
| Refractory to anti-CD38 antibodies | 39 (93) |
| Double-refractory | 36 (86) |
| Triple-refractory | 34 (81) |
| Penta-refractory | 16 (38) |
| Median CAR T-cell dose, million cells (range) | 413.1 (329.7–457.7) |
| Met KarMMa eligibility criteria; n (%) | 12 (29) |
| Safety Outcomes | n (%) |
|---|---|
| Hospitalization | |
| Median hospital stay, days (range) | 8 (6–68) |
| Median intensive care unit stay (n = 4), days (range) | 6 (4–10) |
| CRS | |
| Any | 34 (81) |
| Grade 1 | 29 (69) |
| Grade 2 | 5 (12) |
| Grade 3 | 0 (-) |
| Grade 4 | 0 (-) |
| Median time to maximum severity, days (range) | 1 (0–13) |
| Median duration, days (range) | 2.5 (1–6) |
| Neurotoxicity | |
| Any | 4 (10) |
| Grade 1 | 2 (5) |
| Grade 2 | 0 (-) |
| Grade 3 | 1 (2) |
| Grade 4 | 1 (2) |
| Median time to maximum severity, days (range) | 3 (1–5) |
| Median duration, days (range) | 1.5 (1–6) |
| Supportive care for CRS and neurotoxicity * | |
| Tocilizumab | 34 (81) |
| Corticosteroid | 14 (33) |
| Anakinra | 1 (2) |
| Maximum ferritin, median ng/mL (range) | 816 (61–39,188) |
| Maximum CRP, median mg/dL (range) | 5.8 (0.6–32.8) |
| Any infection | 17 (41) |
| Hematologic toxicity * | |
| Neutropenia | |
| Any | 41 (100) |
| Grade 1 | 2 (5) |
| Grade 2 | 6 (15) |
| Grade ≥ 3 | 33 (81) |
| Anemia | |
| Any | 41 (100) |
| Grade 1 | 6 (14) |
| Grade 2 | 18 (43) |
| Grade ≥ 3 | 17 (41) |
| Thrombocytopenia | |
| Any | 40 (98) |
| Grade 1 | 12 (29) |
| Grade 2 | 7 (17) |
| Grade ≥ 3 | 21 (51) |
| Supportive care for hematologic toxicity † | |
| Granulocyte colony stimulating factor | 34 (81) |
| Thrombopoietin agonist | 1 (2) |
| Clinical Outcomes | n(%) |
| Best overall response by day 90 | |
| Complete response or better | 16 (38) |
| Very good partial response | 2 (5) |
| Partial response | 13 (31) |
| Stable disease/minor response | 5 (12) |
| Progressive disease | 5 (12) |
| Died or progressed before day 90 | 1 (2) |
| Median time to first response, days (range) | 29 (27–125) |
| Median time to complete response or better, days (range) | 28 (27–91) |
| Minimal residual disease negativity at 10−6 | 12 (29) |
| Baseline n = 42 | D0 n = 34 | D7 n = 36 | D14 n = 38 | D21 n = 39 | D30 n = 39 | D60 n = 40 | D90 n = 40 | |
|---|---|---|---|---|---|---|---|---|
| FACT-G | ||||||||
| Overall HRQOL | 79.2 (12.4) | 78.4 (14.0) | 79.3 (9.0) | 77.5 (11.6) | 77.6 (14.1) | 77.8 (15.7) | 83.2 (16.7) | 83.8 (15.9) |
| Functional well-being | 16.8 (5.8) | 14.8 (5.1) | 14.8 (4.8) | 13.5 (5.3) | 14.4 (5.6) | 15.2 (4.9) | 17.3 (5.8) | 18.2 (5.7) |
| Physical well-being | 19.3 (5.3) | 20.3 (4.6) | 20.0 (5.2) | 20.6 (4.6) | 20.2 (4.7) | 20.7 (5.3) | 22.4 (4.5) | 22.0 (5.3) |
| Emotional well-being | 19.0 (3.7) | 20.4 (2.0) | 20.6 (2.7) | 20.5 (2.8) | 20.7 (2.5) | 19.9 (3.6) | 20.5 (3.2) | 20.5 (3.7) |
| Social well-being | 24.1 (3.5) | 23.9 (5.8) | 24.0 (3.5) | 22.8 (5.2) | 23.1 (4.1) | 23.5 (4.1) | 24.5 (3.2) | 23.7 (3.8) |
| PROMIS-29+2 Profile v2.1 | ||||||||
| Fatigue | 53.6 (8.6) | 54.8 (7.9) | 58.8 (8.9) | 56.2 (9.5) | 56.4 (8.3) | 55.7 (8.0) | 51.7 (9.9) | 51.1 (9.6) |
| Pain interference | 55.0 (10.0) | 55.3 (10.2) | 54.1 (9.4) | 54.7 (11.4) | 54.5 (10.5) | 53.8 (10.8) | 52.3 (9.9) | 52.1 (9.7) |
| Sleep disturbance | 50.3 (8.5) | 52.3 (8.1) | 52.1 (10.5) | 50.6 (11.1) | 49.3 (10.1) | 49.7 (10.3) | 47.9 (9.3) | 46.7 (10.3) |
| Depression | 46.4 (6.6) | 45.4 (5.7) | 45.2 (5.9) | 46.7 (6.5) | 45.9 (7.0) | 46.6 (5.9) | 45.1 (6.4) | 45.2 (7.1) |
| Anxiety | 47.4 (6.4) | 47.8 (7.3) | 45.4 (6.4) | 45.9 (7.0) | 45.5 (7.3) | 46.4 (7.3) | 44.3 (6.4) | 45.0 (6.2) |
| Global pain | 3.7 (2.5) | 3.9 (2.4) | 3.1 (2.7) | 3.3 (3.0) | 3.0 (2.6) | 2.8 (2.6) | 3.1 (2.6) | 2.9 (2.4) |
| Performance status | 41.9 (9.4) | 40.4 (8.6) | 37.9 (6.6) | 38.5 (7.9) | 38.3 (8.8) | 38.6 (8.4) | 41.7 (10.2) | 41.5 (8.5) |
| Cognitive function | 50.7 (9.2) | 52.1 (6.7) | 51.1 (7.0) | 51.3 (7.0) | 50.3 (7.9) | 52.4 (7.5) | 53.1 (9.2) | 55.2 (8.0) |
| Social function | 47.0 (10.5) | 48.0 (8.9) | 46.1 (7.9) | 45.3 (7.7) | 45.0 (7.7) | 45.7 (7.9) | 49.2 (10.2) | 50.1 (9.5) |
| Overall symptom burden | 3.2 (0.8) | 3.1 (0.9) | 3.5 (0.6) | 3.0 (0.8) | 3.0 (0.8) | 2.9 (0.8) | 2.9 (0.9) | 2.8 (0.9) |
| Daily Measure | D0 n = 34 | D1 n = 21 | D2 n = 29 | D3 n = 32 | D4 n = 28 | D5 n = 35 | D6 n = 36 | |
| FACT-G7 HRQOL | 16.1 (4.6) | 16.6 (5.0) | 17.1 (3.6) | 16.2 (3.7) | 16.0 (3.9) | 17.1 (3.3) | 17.9 (3.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, L.B.; Gudenkauf, L.M.; Li, X.; De Avila, G.; Peres, L.C.; Kirtane, K.; Gonzalez, B.D.; Hoogland, A.I.; Nguyen, O.; Rodriguez, Y.; et al. Patient-Reported Outcomes among Multiple Myeloma Patients Treated with Standard of Care Idecabtagene Vicleucel. Cancers 2023, 15, 4711. https://doi.org/10.3390/cancers15194711
Oswald LB, Gudenkauf LM, Li X, De Avila G, Peres LC, Kirtane K, Gonzalez BD, Hoogland AI, Nguyen O, Rodriguez Y, et al. Patient-Reported Outcomes among Multiple Myeloma Patients Treated with Standard of Care Idecabtagene Vicleucel. Cancers. 2023; 15(19):4711. https://doi.org/10.3390/cancers15194711
Chicago/Turabian StyleOswald, Laura B., Lisa M. Gudenkauf, Xiaoyin Li, Gabriel De Avila, Lauren C. Peres, Kedar Kirtane, Brian D. Gonzalez, Aasha I. Hoogland, Oanh Nguyen, Yvelise Rodriguez, and et al. 2023. "Patient-Reported Outcomes among Multiple Myeloma Patients Treated with Standard of Care Idecabtagene Vicleucel" Cancers 15, no. 19: 4711. https://doi.org/10.3390/cancers15194711
APA StyleOswald, L. B., Gudenkauf, L. M., Li, X., De Avila, G., Peres, L. C., Kirtane, K., Gonzalez, B. D., Hoogland, A. I., Nguyen, O., Rodriguez, Y., Baz, R. C., Shain, K. H., Alsina, M., Locke, F. L., Freeman, C., Castaneda Puglianini, O., Nishihori, T., Liu, H., Blue, B., ... Hansen, D. K. (2023). Patient-Reported Outcomes among Multiple Myeloma Patients Treated with Standard of Care Idecabtagene Vicleucel. Cancers, 15(19), 4711. https://doi.org/10.3390/cancers15194711