Morphologic Severity of Atypia Is Predictive of Lung Cancer Diagnosis
Abstract
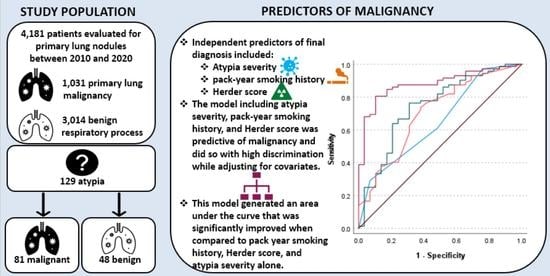
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Data Analytic Plan
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zeng, X.T.; Xing, F.; Fan, N.; Liao, M.Y. The diagnostic accuracy of ct-guided percutaneous core needle biopsy and fine needle aspiration in pulmonary lesions: A meta-analysis. Clin. Radiol. 2016, 71, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; McCrory, D.C. Performance characteristics of different modalities for diagnosis of suspected lung cancer. Chest 2003, 123, 115S–128S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjah, F.; Monsell, S.E.; Gould, M.K.; Smith-Bindman, R.; Banegas, M.P.; Heagerty, P.J.; Keast, E.M.; Ramaprasan, A.; Schoen, K.; Brewer, E.G.; et al. Association of the intensity of diagnostic evaluation with outcomes in incidentally detected lung nodules. JAMA Intern Med. 2021, 181, 480–489. [Google Scholar] [CrossRef]
- Bradley, S.H.; Kennedy, M.P.T.; Neal, R.D. Recognising lung cancer in primary care. Adv. Ther. 2019, 36, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triplette, M.; Kross, E.K.; Mann, B.A.; Elmore, J.G.; Slatore, C.G.; Shahrir, S.; Romine, P.E.; Frederick, P.D.; Crothers, K. An assessment of primary care and pulmonary provider perspectives on lung cancer screening. Ann. Am. Thorac. Soc. 2018, 15, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bilfinger, T.V.; Albano, D.; Perwaiz, M.; Keresztes, R.; Nemesure, B. Survival outcomes among lung cancer patients treated using a multidisciplinary team approach. Clin. Lung Cancer 2018, 19, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, A.; Jassem, J. Multidisciplinary team care in advanced lung cancer. Transl. Lung Cancer Res. 2020, 9, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Coory, M.; Gkolia, P.; Yang, I.A.; Bowman, R.V.; Fong, K.M. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer 2008, 60, 14–21. [Google Scholar] [CrossRef]
- Barletta, J.A.; Yeap, B.Y.; Chirieac, L.R. Prognostic significance of grading in lung adenocarcinoma. Cancer 2010, 116, 659–669. [Google Scholar] [CrossRef]
- Prindiville, S.A.; Byers, T.; Hirsch, F.R.; Franklin, W.A.; Miller, Y.E.; Vu, K.O.; Wolf, H.J.; Baro, A.E.; Shroyer, K.R.; Zeng, C.; et al. Sputum cytological atypia as a predictor of incident lung cancer in a cohort of heavy smokers with airflow obstruction. Cancer Epidemiol. Biomark. Prev. 2003, 12, 987–993. [Google Scholar]
- Albano, D.; Santore, L.A.; Bilfinger, T.; Feraca, M.; Novotny, S.; Nemesure, B. Clinical implications of “atypia” on biopsy: Possible precursor to lung cancer? Curr. Oncol. 2021, 28, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Byers, T.; Wolf, H.J.; Franklin, W.A.; Braudrick, S.; Merrick, D.T.; Shroyer, K.R.; Hirsch, F.R.; Zeng, C.; Barón, A.E.; Bunn, P.A.; et al. Sputum cytologic atypia predicts incident lung cancer: Defining latency and histologic specificity. Cancer Epidemiol. Biomark. Prev. 2008, 17, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Herder, G.J.; van Tinteren, H.; Golding, R.P.; Kostense, P.J.; Comans, E.F.; Smit, E.F.; Hoekstra, O.S. Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18f-fluorodeoxyglucose positron emission tomography. Chest 2005, 128, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Silverstein, M.D.; Ilstrup, D.M.; Schleck, C.D.; Edell, E.S. The probability of malignancy in solitary pulmonary nodules: Application to small radiologically indeterminate nodules. Arch. Intern. Med. 1997, 157, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, A.; Malhotra, P.; Thygesen, H.; Plant, P.K.; Vaidyanathan, S.; Karthik, S.; Scarsbrook, A.; Callister, M.E. Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer. 2015, 89, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Grgic, A.; Yüksel, Y.; Gröschel, A.; Schäfers, H.-J.; Sybrecht, G.W.; Kirsch, C.-M.; Hellwig, D. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1087–1094. [Google Scholar] [CrossRef]
- Hadique, S.; Jain, P.; Hadi, Y.; Baig, A.; Parker, J.E. Utility of FDG PET/CT for assessment of lung nodules identified during low dose computed tomography screening. BMC Med. Imaging 2020, 20, 69. [Google Scholar] [CrossRef]
- Groheux, D.; Quere, G.; Blanc, E.; Lemarignier, C.; Vercellino, L.; de Margerie-Mellon, C.; Merlet, P.; Querellou, S. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn. Interv. Imaging 2016, 97, 1003–1017. [Google Scholar] [CrossRef]
- Cronin, P.; Dwamena, B.A.; Kelly, A.M.; Carlos, R.C. Solitary pulmonary nodules: Meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008, 246, 772–782. [Google Scholar] [CrossRef]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the Eighth Edition of TNM Staging of Non-Small Cell Lung Cancer. Radiographics 2018, 38, 2134–2149. [Google Scholar] [CrossRef]
- Fan, Y.; Su, Z.; Wei, M.; Liang, H.; Jiang, Y.; Li, X.; Meng, Z.; Wang, Y.; Pan, H.; Song, J.; et al. Long-term lung cancer risk associated with sputum atypia: A 27-year follow-up study of an occupational lung screening cohort in yunnan, china. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Tindle, H.A.; Duncan, M.S.; Greevy, R.A.; Vasan, R.S.; Kundu, S.; Massion, P.P.; Freiberg, M.S. Lifetime Smoking History and Risk of Lung Cancer: Results From the Framingham Heart Study. J. Natl. Cancer Inst. 2018, 110, 1201–1207. [Google Scholar] [CrossRef]
- Modin, H.E.; Fathi, J.T.; Gilbert, C.R.; Wilshire, C.L.; Wilson, A.K.; Aye, R.W.; Farivar, A.S.; Louie, B.E.; Vallières, E.; Gorden, J.A. Pack-Year Cigarette Smoking History for Determination of Lung Cancer Screening Eligibility. Comparison of the Electronic Medical Record versus a Shared Decision-making Conversation. Ann. Am. Thorac. Soc. 2017, 14, 1320–1325. [Google Scholar] [CrossRef]
- Gupta, S.; Jacobson, F.L.; Kong, C.Y.; Hammer, M.M. Performance of Lung Nodule Management Algorithms for Lung-RADS Category 4 Lesions. Acad. Radiol. 2021, 28, 1037–1042. [Google Scholar] [CrossRef]
- Ordidge, K.L.; Gandy, N.; Arshad, M.A.; Wallitt, K.; Soneji, N.; Khan, S.; Barwick, T.D. Interobserver agreement of the visual Herder scale for the assessment of solitary pulmonary nodules on 18F Fluorodeoxyglucose PET/computed tomography. Nucl. Med. Commun. 2020, 41, 235–240. [Google Scholar] [CrossRef]
- Gould, M.K.; Sanders, G.D.; Barnett, P.G.; Rydzak, C.E.; MacLean, C.C.; McClellan, M.B.; Owens, U.K. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann. Intern. Med. 2003, 138, 724–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez, E.R.; Caverly, T.J.; Zhang, S.; Glickman, M.E.; Qian, S.X.; Boudreau, J.H.; Miller, D.R.; Slatore, C.G.; Wiener, R.S. Factors associated with declining lung cancer screening after discussion with a physician in a cohort of us veterans. JAMA Netw. Open 2022, 5, e2227126. [Google Scholar] [CrossRef]
- Reese, T.J.; Schlechter, C.R.; Kramer, H.; Kukhareva, P.; Weir, C.R.; Del Fiol, G.; Caverly, T.; Hess, R.; Flynn, M.C.; Taft, T.; et al. Implementing lung cancer screening in primary care: Needs assessment and implementation strategy design. Transl. Behav. Med. 2021, 12, 187–197. [Google Scholar] [CrossRef]


| Total | Cancer | Benign | ||
|---|---|---|---|---|
| Total n (%) | 129 (100%) | 81 (62.8%) | 48 (37.2%) | |
| Age (M + SD) | 66.53 ± 10.65 | 67.10 ± 9.16 | 65.56 ± 12.82 | |
| Female n (%) | 63 (48.8%) | 42 (51.9%) | 21 (43.8%) | |
| Pack years (M + SD) | 40.51 ± 35.94 | 47.33 ± 37.83 ** | 23.59 ± 23.83 ** | |
| Atypia severityn (%) | Favor reactive | 15 (11.6%) | 2 (2.5%) *** | 13 (27.1%) *** |
| Mild | 43 (33.3%) | 29 (35.8%) | 14 (29.2%) | |
| Moderate | 45 (34.9%) | 26 (32.1%) | 19 (39.6%) | |
| Severe | 26 (20.2%) | 24 (29.6%) *** | 2 (4.2%) *** | |
| Modified Herder score (M ± SD) | 0.600 ± 0.331 | 0.690 ± 0.290 *** | 0.446 ± 0.343 *** | |
| Qualitative SUVn (%) | Not performed | 29 (22.5%) | 7 (8.6%) *** | 22 (45.8%) *** |
| Absent (<1) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Faint (1–2.5) | 34 (26.4%) | 21 (25.9%) | 13 (27.1%) | |
| Moderate (2.5–4) | 27 (20.9%) | 17 (21.0%) | 10 (20.8%) | |
| Intense (>4) | 39 (30.2%) | 36 (44.4%) *** | 3 (6.3%) *** | |
| Maximum SUV (M ± SD) | 5.17 ± 5.27 | 6.01 ± 5.81 *** | 2.78 ± 1.88 *** | |
| Sampling methodn (%) | BAL | 16 (12.4%) | 5 (6.2%) ** | 11 (22.9%) ** |
| Brushing | 8 (6.2%) | 5 (6.2%) | 3 (6.3%) | |
| IR | 9 (7.0%) | 3 (3.7%) | 6 (12.5%) | |
| Bronch | 27 (20.9%) | 16 (19.8%) | 11 (22.9%) | |
| FNA | 63 (48.8%) | 47 (58.0%) ** | 16 (33.3%) ** | |
| Pleural fluid | 6 (4.7%) | 5 (6.2%) | 1 (2.1%) | |
| Final Diagnosis | Atypia Severity | Sensitivity | Specificity | False Positives | False Negatives |
|---|---|---|---|---|---|
| Cancer | Favor reactive changes | 2.5% | 72.9% | 13 | 79 |
| Mild | 35.8% | 70.8% | 14 | 52 | |
| Moderate | 32.1% | 60.4% | 19 | 55 | |
| Severe | 29.6% | 95.8% | 2 | 57 | |
| Benign respiratory process | Favor reactive changes | 27.1% | 97.5% | 2 | 35 |
| Mild | 29.2% | 64.2% | 29 | 34 | |
| Moderate | 39.6% | 67.9% | 26 | 29 | |
| Severe | 4.2% | 70.4% | 24 | 46 |
| Variable | OR (95% CI) | c (95% CI) |
|---|---|---|
| Pack-year-smoking history | 0.97 (0.95, 0.99) * | 0.71 (0.59, 0.82) *** |
| Modified Herder score | 0.05 (0.01, 0.28) *** | 0.74 (0.63, 0.85) *** |
| Atypia severity | - | 0.65 (0.54, 0.77) *** |
| Mild | 46.66 (4.11, 530.45) ** | - |
| Moderate | 3.55 (0.56, 22.56) | - |
| Severe | 8.80 (1.39, 55.66) * | - |
| Model including Pack-year-smoking history, Modified Herder score, and Atypia severity | - | 0.88 (0.81, 0.95) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santore, L.A.; Novotny, S.; Tseng, R.; Patel, M.; Albano, D.; Dhamija, A.; Tannous, H.; Nemesure, B.; Shroyer, K.R.; Bilfinger, T. Morphologic Severity of Atypia Is Predictive of Lung Cancer Diagnosis. Cancers 2023, 15, 397. https://doi.org/10.3390/cancers15020397
Santore LA, Novotny S, Tseng R, Patel M, Albano D, Dhamija A, Tannous H, Nemesure B, Shroyer KR, Bilfinger T. Morphologic Severity of Atypia Is Predictive of Lung Cancer Diagnosis. Cancers. 2023; 15(2):397. https://doi.org/10.3390/cancers15020397
Chicago/Turabian StyleSantore, Lee Ann, Samantha Novotny, Robert Tseng, Mit Patel, Denise Albano, Ankit Dhamija, Henry Tannous, Barbara Nemesure, Kenneth R. Shroyer, and Thomas Bilfinger. 2023. "Morphologic Severity of Atypia Is Predictive of Lung Cancer Diagnosis" Cancers 15, no. 2: 397. https://doi.org/10.3390/cancers15020397
APA StyleSantore, L. A., Novotny, S., Tseng, R., Patel, M., Albano, D., Dhamija, A., Tannous, H., Nemesure, B., Shroyer, K. R., & Bilfinger, T. (2023). Morphologic Severity of Atypia Is Predictive of Lung Cancer Diagnosis. Cancers, 15(2), 397. https://doi.org/10.3390/cancers15020397