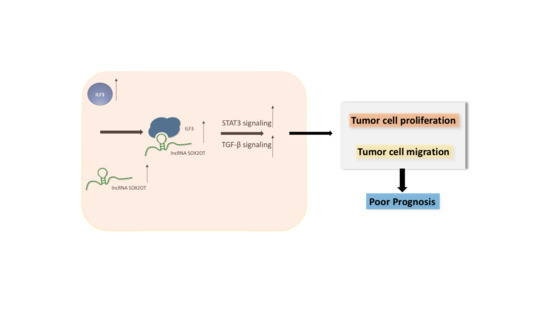
SOX2-OT Binds with ILF3 to Promote Head and Neck Cancer Progression by Modulating Crosstalk between STAT3 and TGF-β Signaling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HNSCC Patients and Clinical Samples
2.2. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.3. Cell Culture and Transfection
2.4. Cell Proliferation and Formation Assays
2.5. Flow Cytometry
2.6. Western Blot Analysis
2.7. Transwell Assay
2.8. Mouse Xenograft Experiments
2.9. RNA Fluorescence In Situ Hybridization (FISH)
2.10. RNA Pulldown Assay
2.11. RNA Immunoprecipitation Assay
2.12. Statistical Analysis
3. Results
3.1. SOX2-OT Overexpression Predicts Poor Overall Survival in HNSCC
3.2. SOX2-OT Promotes Cell Progression and Migration, Meanwhile Inhibits Apoptosis in HNSCC
3.3. SOX2-OT Facilitates HNSCC Xenograft Growth
3.4. SOX2-OT Binds with ILF3 to Exert Function in HNSCC
3.5. SOX2-OT Promoted HNSCC Cell Progression and Migration Depending on ILF3
3.6. SOX2-OT Regulates the Crosstalk between STAT3 and TGF-β by ILF3 in HNSCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GCN5 | General control non-depressible 5 |
| H3K27 | Lysine 27 on histone H3 |
| HIF1A | Hypoxia Inducible Factor 1 Subunit Alpha |
| POP1 | Processing of Precursor 1 |
| ILF3 | Interleukin enhancer-binding factor 3 |
| ERp57 | Protein Disulfide Isomerase Family A Member 3 |
References
- Luo, X.; Qiu, Y.; Jiang, Y.; Chen, F.; Jiang, L.; Zhou, Y.; Dan, H.; Zeng, X.; Lei, Y.L.; Chen, Q. Long non-coding RNA implicated in the invasion and metastasis of head and neck cancer: Possible function and mechanisms. Mol. Cancer 2018, 17, 14. [Google Scholar] [CrossRef]
- Liu, S.J.; Dang, H.X.; Lim, D.A.; Feng, F.Y.; Maher, C.A. Long noncoding RNAs in cancer metastasis. Nat. Rev. Cancer 2021, 21, 446–460. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, Z.; Wang, R.; Han, Y.; Wang, C.; Yang, F.; Han, Y.; Liang, H.; Qi, L.; Wang, C. Identification of driver copy number alterations in diverse cancer types and application in drug repositioning. Mol. Oncol. 2017, 11, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, H.L.; Kim, B.J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018, 50, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yang, J.; Qiu, Q.; Li, X.; Lu, Y. LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J. Hematol. Oncol. 2021, 14, 112. [Google Scholar] [CrossRef]
- Yan, T.; Shen, C.; Jiang, P.; Yu, C.; Guo, F.; Tian, X.; Zhu, X.; Lu, S.; Han, B.; Zhong, M. Risk SNP-induced lncRNA-SLCC1 drives colorectal cancer through activating glycolysis signaling. Signal Transduct. Target. Ther. 2021, 6, 70. [Google Scholar] [CrossRef]
- Xiao-Dan, Z.; Guo-Wei, H.; Ying-Hua, X.; Jian-Zhong, H.; Jin-Cheng, G.; Xiu, E.X.; Lian-Di, L.; Yang-Min, X.; Yong-Mei, S.; En-Min, L. The interaction of lncRNA EZR-AS1 with SMYD3 maintains overexpression of EZR in ESCC cells. Nucleic Acids Res. 2017, 4, 1793–1809. [Google Scholar]
- Ma, H.; Chang, H.; Yang, W.; Lu, Y.; Hu, J.; Jin, S. A novel IFNα-induced long noncoding RNA negatively regulates immunosuppression by interrupting H3K27 acetylation in head and neck squamous cell carcinoma. Mol. Cancer 2020, 19, 4. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Z.; Feng, L.; Yang, Y.; Tan, C.; Shi, Q.; Lian, M.; He, S.; Ma, H.; Fang, J. LncRNA MIR31HG targets HIF1A and P21 to facilitate head and neck cancer cell proliferation and tumorigenesis by promoting cell-cycle progression. Mol. Cancer 2018, 17, 162. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, H.; Tong, T.; Xie, F.; Qin, X.; Wang, X.; Chen, W.; Zhang, J. Long noncoding RNA lnc-POP1-1 upregulated by VN1R5 promotes cisplatin resistance in head and neck squamous cell carcinoma through interaction with MCM5. Mol. Ther. 2022, 30, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, R.; Lian, M.; Ma, H.; He, N.; Liu, H.; Wang, H.; Fang, J. Integrated Analysis of Long Noncoding RNA and mRNA Expression Profile in Advanced Laryngeal Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0169232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, S.; Chen, D. SOX2-OT induced by PAI-1 promotes triple-negative breast cancer cells metastasis by sponging miR-942-5p and activating PI3K/Akt signaling. Cell. Mol. Life Sci. 2022, 79, 59. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Solorio, A.M.; Peralta-Arrieta, I.; López, L.A.; Hernández-Cigala, N.; Milla, C.M.; Quintero, B.O.; Cárdenas, R.C.; Villegas, P.P.; Villanueva, E.R.; Iriarte, C.G.T. LncRNA SOX2-OT regulates AKT/ERK and SOX2/GLI-1 expression, hinders therapy and worsens clinical prognosis in malignant lung diseases. Mol. Oncol. 2021, 15, 1110–1129. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Dong, L.; Jin, H.; Li, H.; Sun, M.; Li, J. Exosome long non-coding RNA SOX2-OT contributes to ovarian cancer malignant progression by miR-181b-5p/SCD1 signaling. Aging 2021, 13, 23726–23738. [Google Scholar] [CrossRef]
- Liu, J.; Shang, G. The Roles of Noncoding RNAs in the Development of Osteosarcoma Stem Cells and Potential Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 773038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Lian, J.; Zhang, H.; Yu, B.; Zhang, M.; Wei, F.; Wu, J.; Jiang, J.; Jia, Y. The lncRNA PVT1 regulates nasopharyngeal carcinoma cell proliferation via activating the KAT2A acetyltransferase and stabilizing HIF-1α. Nat. Publ. Group 2020, 27, 695–710. [Google Scholar] [CrossRef]
- Wang, H.; Lin, X.; Li, J.; Zeng, G.; Xu, T. Long Noncoding RNA SOX2-OT Aggravates Doxorubicin-Induced Apoptosis of Cardiomyocyte by Targeting miR-942-5p/DP5. Drug Des. Dev. Ther. 2021, 15, 481–492. [Google Scholar] [CrossRef]
- Huarte, M. The emerging role of lncRNAs in cancer. Nat. Med. 2015, 21, 1253. [Google Scholar] [CrossRef]
- Raju, G.; Pavitra, E.; Bandaru, S.; Varaprasad, G.; Nagaraju, G.; Malla, R.; Huh, Y.; Han, Y. HOTAIR: A potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol. Cancer 2023, 65, 65. [Google Scholar] [CrossRef]
- Marchese, F.; Huarte, P. Long non-coding RNAs and chromatin modifiers: Their place in the epigenetic code. Epigenetics 2014, 9, 21–26. [Google Scholar] [CrossRef]
- Amirinejad, R.; Rezaei, M.; Shirvani-Farsani, Z. An update on long intergenic noncoding RNA p21: A regulatory molecule with various significant functions in cancer. Cell Biosci. 2020, 10, 82. [Google Scholar] [CrossRef]
- Puvvula, P.K.; Desetty, R.D.; Pineau, P.; Marchio, A.; Bischof, O. Long noncoding RNA PANDAR and scaffold-attachment-factor SAFA control senescence entry and exit. Nat. Commun. 2014, 5, 5323. [Google Scholar] [CrossRef]
- Guo, K.; Qian, K.; Shi, Y.; Sun, T.; Wang, Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Lang, C.; Yin, C.; Lin, K.; Li, Y.; Yang, Q.; Wu, Z.; Du, H.; Ren, D.; Dai, Y.; Peng, X. m6A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin. Transl. Med. 2021, 11, e426. [Google Scholar] [CrossRef]
- Vrakas, C.N.; Herman, A.B.; Ray, M.; Kelemen, S.E.; Scalia, R.; Autieri, M.V. RNA stability protein ILF3 mediates cytokine-induced angiogenesis. FASEB J. 2018, 33, 3304–3316. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, H.; Ding, L.; Zhu, P.; Yu, L. Regulation of cell cycle of hepatocellular carcinoma by NF90 through modulation of cyclin E1 mRNA stability. Oncogene 2015, 34, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lu, Y.Y.; Noh, H.; Hong, S.; Dong, Z.; Ding, H.F.; Su, S.B.; Huang, S. Interleukin enhancer-binding factor 3 promotes breast tumor progression by regulating sustained urokinase-type plasminogen activator expression. Oncogene 2013, 32, 3933–3943. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.X.; Nie, Z.Y.; Wen, Y.; Shi, Y.H. Upregulation of ERp57 promotes clear cell renal cell carcinoma progression by initiating a STAT3/ILF3 feedback loop. J. Exp. Clin. Cancer Res. 2019, 38, 439. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, C. Regulation of EMT by STAT3 in gastrointestinal cancer (Review). Int. J. Oncol. 2017, 50, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Wang, L.; Wei, H.; Zhang, T.; Zhang, L.; Lin, H.; Zhang, H.; Wang, S. TGF-β1/SH2B3 axis regulates anoikis resistance and EMT of lung cancer cells by modulating JAK2/STAT3 and SHP2/Grb2 signaling pathways. Cell Death Dis. 2022, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, T.; Wu, J.C.; Luo, S.Z.; Xu, M.Y. STAT3 aggravates TGF-β1-induced hepatic epithelial-to-mesenchymal transition and migration. Biomed. Pharmacother. 2017, 98, 214–221. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Cases | SOX2-OT Relative Expression | p-Value | |
|---|---|---|---|---|
| Low | High | |||
| Age years | 0.301 | |||
| <60 | 28 | 16 | 12 | |
| ≥60 | 29 | 14 | 15 | |
| T category | 0.705 | |||
| T1–T2 | 34 | 16 | 18 | |
| T3–T4 | 23 | 12 | 11 | |
| Lymph-node metastasis | 0.698 | |||
| N0 | 44 | 21 | 23 | |
| N1–N2 | 13 | 7 | 6 | |
| Clinical stage | 0.903 | |||
| I–II | 31 | 15 | 16 | |
| III–IV | 26 | 13 | 13 | |
| Differentiation | 0.031 | |||
| Well | 21 | 15 | 6 | |
| Moderate | 32 | 11 | 21 | |
| Poor | 4 | 2 | 2 | |
| Variable | Univariate | Multivariate | p-Value | |
|---|---|---|---|---|
| Hazard Ratio | 95% CI | |||
| Age years | ||||
| <60 vs. ≥60 | 0.302 | |||
| T category | ||||
| T1–T2 vs. T3–T4 | 0.644 | |||
| Lymph-node metastasis | ||||
| N0 vs. N1–N2 | 0.769 | |||
| Clinical stage | ||||
| I–II vs. III–IV | 0.989 | |||
| Differentiation | ||||
| Well vs. Moderate–Poor | 0.087 | 2.507 | 0.716–8.782 | 0.151 |
| SOX2-OT expression | ||||
| High vs. Low | 0.023 | 3.811 | 1.006–14.444 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, Y.; Wang, L.; Shi, Q.; Ma, H.; He, S.; Feng, L.; Fang, J. SOX2-OT Binds with ILF3 to Promote Head and Neck Cancer Progression by Modulating Crosstalk between STAT3 and TGF-β Signaling. Cancers 2023, 15, 5766. https://doi.org/10.3390/cancers15245766
Wang R, Yang Y, Wang L, Shi Q, Ma H, He S, Feng L, Fang J. SOX2-OT Binds with ILF3 to Promote Head and Neck Cancer Progression by Modulating Crosstalk between STAT3 and TGF-β Signaling. Cancers. 2023; 15(24):5766. https://doi.org/10.3390/cancers15245766
Chicago/Turabian StyleWang, Ru, Yifan Yang, Lingwa Wang, Qian Shi, Hongzhi Ma, Shizhi He, Ling Feng, and Jugao Fang. 2023. "SOX2-OT Binds with ILF3 to Promote Head and Neck Cancer Progression by Modulating Crosstalk between STAT3 and TGF-β Signaling" Cancers 15, no. 24: 5766. https://doi.org/10.3390/cancers15245766
APA StyleWang, R., Yang, Y., Wang, L., Shi, Q., Ma, H., He, S., Feng, L., & Fang, J. (2023). SOX2-OT Binds with ILF3 to Promote Head and Neck Cancer Progression by Modulating Crosstalk between STAT3 and TGF-β Signaling. Cancers, 15(24), 5766. https://doi.org/10.3390/cancers15245766