Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Small EV Isolation from Blood Plasma
2.3. Quantification of sEV Concentration and Size
2.4. Statistical Analysis
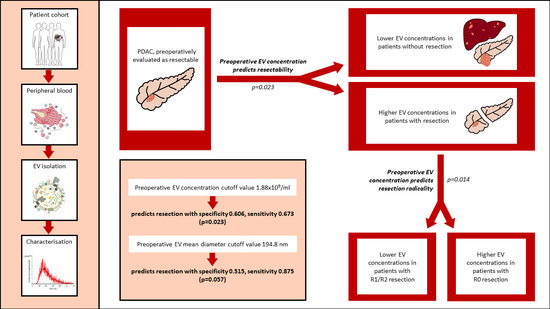
3. Results
3.1. Patient Characteristics
3.2. Patients’ Plasma Small Extracellular Vesicle Characteristics
3.3. Comparison of Plasma Small Extracellular Vesicle Characteristics between Patients with and without Resection
3.4. Association between Radicality of Resection and Plasma sEV Characteristics
3.5. Association between Plasma sEV Characteristics and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. New Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.B.; Gurusamy, K.S.; Takwoingi, Y.; Kalia, A.; Davidson, B.R. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst. Rev. 2013, 11, D009323. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Larghi, A.; Correale, L.; Ricci, R.; Abdulkader, I.; Monges, G.; Iglesias-Garcia, J.; Giovannini, M.; Attili, F.; Vitale, G.; Hassan, C.; et al. Interobserver agreement and accuracy of preoperative endoscopic ultrasound-guided biopsy for histological grading of pancreatic cancer. Endoscopy 2015, 47, 308–314. [Google Scholar] [CrossRef]
- Groot, V.P.; Gemenetzis, G.; Blair, A.B.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Rinkes, I.H.M.B.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef]
- Otandault, A.; Anker, P.; Al Amir Dache, Z.; Guillaumon, V.; Meddeb, R.; Pastor, B.; Pisareva, E.; Sanchez, C.; Tanos, R.; Tousch, G.; et al. Recent advances in circulating nucleic acids in oncology. Ann. Oncol. 2019, 30, 374–384. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Cappelletti, V.; Daidone, M.G.; Smith, L.; Parris, C.; Brosens, L.A.A.; Caruso, M.G.; Cheng, L.; et al. Liquid Biopsy as Surrogate for Tissue for Molecular Profiling in Pancreatic Cancer: A Meta-Analysis Towards Precision Medicine. Cancers 2019, 11, 1152. [Google Scholar] [CrossRef]
- Badovinac, D.; Goričar, K.; Zavrtanik, H.; Petrič, M.; Lavrin, T.; Mavec, N.; Dolžan, V.; Tomažič, A.; Lenassi, M. Plasma Extracellular Vesicle Characteristics Correlate with Tumor Differentiation and Predict Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Surgery with Curative Intent. J. Pers. Med. 2021, 11, 77. [Google Scholar] [CrossRef]
- Rofi, E.; Vivaldi, C.; Del Re, M.; Arrigoni, E.; Crucitta, S.; Funel, N.; Fogli, S.; Vasile, E.; Musettini, G.; Fornaro, L.; et al. The emerging role of liquid biopsy in diagnosis, prognosis and treatment monitoring of pancreatic cancer. Pharmacogenomics 2019, 20, 49–68. [Google Scholar] [CrossRef]
- Massoumi, R.L.; Hines, O.J.; Eibl, G.; King, J.C. Emerging Evidence for the Clinical Relevance of Pancreatic Cancer Exosomes. Pancreas 2019, 48, 1–8. [Google Scholar] [CrossRef]
- González, E.; Falcón-Pérez, J.M. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev. Mol. Diagn. 2015, 15, 907–923. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, J.; Hsu, Y.M.S.; Lyon, C.J.; Ning, B.; Hu, T.Y. Extracellular vesicles as cancer liquid biopsies: From discovery, validation, to clinical application. Lab Chip 2019, 19, 1114–1140. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Hinestrosa, J.P.; Kurzrock, R.; Lewis, J.M.; Schork, N.J.; Schroeder, G.; Kamat, A.M.; Lowy, A.M.; Eskander, R.N.; Perrera, O.; Searson, D.; et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. 2022, 2, 29. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Takahasi, K.; Iinuma, H.; Wada, K.; Minezaki, S.; Kawamura, S.; Kainuma, M.; Ikeda, Y.; Shibuya, M.; Miura, F.; Sano, K. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J. Hepatobiliary. Pancreat. Sci. 2018, 25, 155–161. [Google Scholar] [CrossRef]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. J. Hepatobiliary. Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Alix-Panabières, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef] [Green Version]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S.; Zhang, S.; He, H.Z.; Zheng, S.Y. Extracellular Vesicles as Potential Biomarkers for Early Detection and Diagnosis of Pancreatic Cancer. Biomedicines 2020, 8, 581. [Google Scholar] [CrossRef]
- König, L.; Kasimir-Bauer, S.; Bittner, A.K.; Hoffmann, O.; Wagner, B.; Santos Manvailer, L.F.; Kimmig, R.; Horn, P.A.; Rebmann, V. Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. Oncoimmunology 2017, 7, e1376153. [Google Scholar] [CrossRef] [Green Version]
- Helley, D.; Banu, E.; Bouziane, A.; Banu, A.; Scotte, F.; Fischer, A.M.; Oudard, S. Platelet microparticles: A potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur. Urol. 2009, 56, 479–485. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes. Chromosomes Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef]
- Muller, L.; Muller-Haegele, S.; Mitsuhashi, M.; Gooding, W.; Okada, H.; Whiteside, T.L. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 2015, 4, e1008347. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Kano, M.; Akutsu, Y.; Hanari, N.; Hoshino, I.; Murakami, K.; Usui, A.; Suito, H.; Takahashi, M.; Otsuka, R.; et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 2016, 36, 2535–2543. [Google Scholar] [CrossRef]
- Liu, Q.; Xiang, Y.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Wu, N.; Wu, L.; Yu, Z.; Bai, L.; et al. Plasma exosome levels in non-small-cell lung cancer: Correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018, 22, 267–274. [Google Scholar] [CrossRef]
- Navarro, A.; Molins, L.; Marrades, R.M.; Moises, J.; Viñolas, N.; Morales, S.; Canals, J.; Castellano, J.J.; Ramírez, J.; Monzo, M. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers 2019, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Brierley, J.; Gospodarowicz, M.D.; Wittekind, C.T. TNM Classification of Malignant Tumors International Union Against Cancer, 8th ed.; Wiley: Oxford, UK, 2017; pp. 57–62. [Google Scholar]
- Holcar, M.; Ferdin, J.; Sitar, S.; Tušek-Žnidarič, M.; Dolžan, V.; Plemenitaš, A.; Žagar, E.; Lenassi, M. Enrichment of plasma extracellular vesicles for reliable quantification of their size and concentration for biomarker discovery. Sci. Rep. 2020, 10, 21346. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Štok, U.; Blokar, E.; Lenassi, M.; Holcar, M.; Frank-Bertoncelj, M.; Erman, A.; Resnik, N.; Sodin-Šemrl, S.; Čučnik, S.; Pirkmajer, K.P.; et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells 2020, 9, 1211. [Google Scholar] [CrossRef]
- Levstek, T.; Mlinšek, T.; Holcar, M.; Goričar, K.; Lenassi, M.; Dolžan, V.; Vujkovac, B.; Trebušak Podkrajšek, K. Urinary Extracellular Vesicles and Their miRNA Cargo in Patients with Fabry Nephropathy. Genes 2021, 12, 1057. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Howard, T.J.; Krug, J.E.; Yu, J.; Zyromski, N.J.; Schmidt, C.M.; Jacobson, L.E.; Madura, J.A.; Wiebke, E.A.A.; Lillemoe, K.D. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J. Gastrointest. Surg. 2006, 10, 1338–1346. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Zervos, E.E.; Rosemurgy, A.S.; Al-Saif, O.; Durkin, A.J. Surgical management of early-stage pancreatic cancer. Cancer Control 2004, 11, 23–31. [Google Scholar] [CrossRef]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.A.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S.; et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006, 10, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Reddy, S.; Weiss, M.J.; Manos, L.L.; Cameron, J.L.; Zheng, L.; Herman, J.M.; Hruban, R.H.; Fishman, E.K.; Wolfgang, C.L. Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. AJR. Am. J. Roentgenol. 2015, 204, W37–W42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Li, Y.; Liao, Z.; Wang, Z.; Wang, Z.; Li, Y.; Qian, L.; Zhao, J.; Zong, H.; Kang, B.; et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut 2020, 69, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Rhee, T.M.; Pietrasz, D.; Bachet, J.B.; Laurent-Puig, P.; Kong, S.Y.; Takai, E.; Yachida, S.; Shibata, T.; Lee, J.W.; et al. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Jiang, P.; Chan, K.C.A.; Wong, J.; Cheng, Y.K.Y.; Liang, R.H.S.; Chan, W.K.; Ma, E.S.K.; Chan, S.L.; Cheng, S.H.; et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl. Acad. Sci. USA 2015, 112, E5503–E5512. [Google Scholar] [CrossRef] [Green Version]
- Von Felden, J.; Garcia-Lezana, T.; Schulze, K.; Losic, B.; Villanueva, A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 2020, 69, 2025–2034. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef]
- FDA. FDA Approves Liquid Biopsy Next-Generation Sequencing Companion Diagnostic Test. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-liquid-biopsy-next-generation-sequencing-companion-diagnostic-test (accessed on 10 October 2022).
- Pine, J.K.; Haugk, B.; Robinson, S.M.; Darne, A.; Wilson, C.; Sen, G.; French, J.J.; White, S.A.; Manas, D.M.; Charnley, R.M. Prospective assessment of resection margin status following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma after standardisation of margin definitions. Pancreatology 2020, 20, 537–544. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Foulis, A.K.; Oien, K.A.; Going, J.J.; Glen, P.; Dickson, E.J.; Imrie, C.W.; McKay, C.J.; Carter, R. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2010, 251, 1003–1010. [Google Scholar] [CrossRef]
- Campbell, F.; Smith, R.A.; Whelan, P.; Sutton, R.; Raraty, M.; Neoptolemos, J.P.; Ghaneh, P. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009, 55, 277–283. [Google Scholar] [CrossRef]
- Weyhe, D.; Obonyo, D.; Uslar, V.N.; Stricker, I.; Tannapfel, A. Predictive factors for long-term survival after surgery for pancreatic ductal adenocarcinoma: Making a case for standardized reporting of the resection margin using certified cancer center data. PLoS ONE 2021, 16, e0248633. [Google Scholar] [CrossRef]
- Sedej, I.; Štalekar, M.; Tušek Žnidarič, M.; Goričar, K.; Kojc, N.; Kogovšek, P.; Dolžan, V.; Arnol, M.; Lenassi, M. Extracellular vesicle-bound DNA in urine is indicative of kidney allograft injury. J. Extracell. Vesicles 2022, 11, e12268. [Google Scholar] [CrossRef]



| Variables | Study Patients N = 83 | W/O Resection N = 33 | With Resection N = 50 | p-Value * | |
|---|---|---|---|---|---|
| Sex | Male, N (%) | 51 (61.4) | 22 (66.7) | 29 (58.0) | 0.494 d |
| Female, N (%) | 32 (38.6) | 11 (33.3) | 21 (42.0) | ||
| Age | Years, median (25–75%) | 70 (63–77) | 71 (65–77.5) | 69.5 (61.0–77.0) | 0.536 e |
| ASA score | 1, N (%) | 1 (1.2) {1} | 0 (0.0) | 1 (2.0) {1} | 0.247 d |
| 2, N (%) | 21 (25.6) | 6 (18.2) | 15 (30.6) | ||
| 3, N (%) | 60 (73.2) | 27 (81.8) | 33 (67.3) | ||
| Smoking | No, N (%) | 34 (42.5) {3} | 9 (29.0) {2} | 25 (51.0) {1} | 0.065 d |
| Yes, N (%) | 46 (57.5) | 22 (71.0) | 24 (49.0) | ||
| Alcohol consumption | Never, N (%) | 19 (24.4) {5} | 8 (26.7) {3} | 11 (22.9) {2} | 0.618 d |
| Occasional, N (%) | 40 (51.3) | 15 (50.0) | 25 (52.1) | ||
| Moderate, N (%) | 14 (17.9) | 4 (13.3) | 10 (20.8) | ||
| Heavy, N (%) | 5 (6.4) | 3 (10.0) | 2 (4.2) | ||
| BMI 6 months before surgery | kg/m2, median (25–75%) | 26.4 (23.9–30.7) {4} | 29.0 (25.7–31.4) {3} | 25.7 (22.8–29.7) {1} | 0.014 e |
| BMI at surgery | kg/m2, median (25–75%) | 24.9 (22.0–28.4) {4} | 25.4 (23.8–28.4) {3} | 23.8 (21.5–28.4) {1} | 0.179 e |
| WBC count a | * 109/L, median (25–75%) | 7.5 (5.9–8.6) {3} | 8.1 (5.95–9.15) | 7.3 (5.9–8.4) {3} | 0.200 e |
| CRP a | mg/L, median (25–75%) | 5 (5–21.25) {3} | 10 (5–38.5) | 5 (5–9) {3} | 0.002 e |
| CA19-9 a | median (25–75%) | 1921 (329–10,457.5) {2} | 4532 (354–21,598.25) {1} | 884.0 (231.5–4189.0) | 0.105 e |
| CEA a | median (25–75%) | 3.0 (1.6–5.7) {2} | 3.5 (1.8–8.1) {2} | 2.5 (1.6–4.7) | 0.145 e |
| Preoperatively evaluated tumor size | mm, median (25–75%) | 30 (25–41) {10} | 33 (25–45) {1} | 28 (24.5–36.5) {9} | 0.169 e |
| Distant metastases b | No, N (%) | 63 (75.9) | 16 (48.5) | 47 (94.0) | <0.001 d |
| Yes, N (%) | 20 (24.1) | 17 (51.5) | 3 (6.0) | ||
| Tumor differentiation c | Poor, N (%) | 39 (53.4) {10} | 14 (56.0) {8} | 25 (52.1) {2} | 0.047 d |
| Moderate, N (%) | 32 (43.8) | 11 (44.0) | 21 (43.8) | ||
| Well, N (%) | 2 (2.7) | 0 (0.0) | 2 (4.2) | ||
| Adjuvant chemotherapy † | No, N (%) | 33 (40.7) {2} | 17 (53.1) {1} | 16 (32.7) {1} | 0.105 d |
| Yes, N (%) | 48 (59.3) | 15 (46.9) | 33 (67.3) | ||
| Resection radicality c | R0, N (%) | 24 (49.0) {1} | |||
| R0 (<1 mm), N (%) | 8 (16.3) | ||||
| R1, N (%) | 15 (30.6) | ||||
| R2, N (%) | 2 (4.1) | ||||
| pT stage | 1, N (%) | 1 (1.3) {3} | 0 (0.0) {3} | 1 (2.0) | <0.001 e |
| 2, N (%) | 16 (20.0) | 0 (0.0) | 16 (32.0) | ||
| 3, N (%) | 33 (41.3) | 1 (3.3) | 32 (64.0) | ||
| 4, N (%) | 30 (37.5) | 29 (96.7) | 1 (2.0) | ||
| pN stage | 0, N (%) | 9 (18.0) {33} | 9 (18.0) {33} | ||
| 1, N (%) | 29 (58.0) | 29 (58.0) | |||
| 2, N (%) | 12 (24.0) | 12 (24.0) |
| Study Patients | W/O Resection | With Resection | |||
|---|---|---|---|---|---|
| Small EV Characteristics | Median (25–75%) | Median (25–75%) | Median (25–75%) | p-Value # | |
| Before surgery (N = 82: 33 w/o resection, 49 with resection) | Concentration (N × 109/mL) | 1.97 (1.49–3.10) | 1.66 (1.24–2.46) | 2.14 (1.61–3.29) | 0.023 |
| Mean diameter (nm) | 182.6 (170.1–199) | 195.2 (169.9–213.8) | 181.4 (170–189.1) | 0.057 | |
| After one month (N = 53: 16 w/o resection, 37 with resection) | Concentration (N × 109/mL) | 2.29 (1.61–3.22) | 2.08 (1.46–2.71) | 2.38 (1.71–3.28) | 0.333 |
| Mean diameter (nm) | 188.9 (176.5–204.2) | 196.2 (180.5–218.9) | 185.7 (176.4–196.4) | 0.075 | |
| After six months (N = 43: 9 w/o resection, 34 with resection) | Concentration (N × 109/mL) | 2.68 (1.68–3.30) | 2.15 (1.75–3.06) | 2.78 (1.88–3.32) | 0.385 |
| Mean diameter (nm) | 183.8 (176.6–192.6) | 188.5 (171.1–213) | 181.2 (177.2–191.2) | 0.471 | |
| After 12 months (N = 29: 1 w/o resection, 28 with resection) | Concentration (N × 109/mL) | 2.58 (1.84–3.47) | 3.90 | 2.54 (1.79–3.42) | * |
| Mean diameter (nm) | 177.2 (166–191.3) | 149.7 | 177.4 (170.7–191.9) | * |
| Small EV Characteristics | HR (95% CI) * | p-Value | HR (95% CI) adj * | p-Value adj | |
|---|---|---|---|---|---|
| Study Patients | Concentration (N × 109/mL) | 1.00 (1.00–1.00) | 0.069 | 1.00 (1.00–1.00) | 0.599 |
| Mean diameter (nm) | 1.10 (0.98–1.24) | 0.121 | 0.92 (0.80–1.05) | 0.224 | |
| w/o Resection | Concentration (N × 109/mL) | 1.00 (1.00–1.00) | 0.895 | 1.00 (1.00–1.00) | 0.714 |
| Mean diameter (nm) | 0.81 (0.68–0.97) | 0.021 | 0.85 (0.71–1.01) | 0.065 | |
| With Resection | Concentration (N × 109/mL) | 1.00 (1.00–1.00) | 0.407 | 1.00 (1.00–1.00) | 0.535 |
| Mean diameter (nm) | 1.10 (0.89–1.34) | 0.381 | 1.07 (0.86–1.32) | 0.555 |
| Small EV Characteristics | Survival Months, Median (25–75%) | HR (95% CI) | p-Value | HR (95% CI) adj | p-Value adj | |
|---|---|---|---|---|---|---|
| Concentration (N × 109/mL) | <1.88 × 109/mL | 7.8 (3.8–13.8) | Reference | Reference | ||
| >1.88 × 109/mL | 16.1 (7.7–28.3) | 0.54 (0.32–0.90) | 0.018 | 0.74 (0.41–1.35) | 0.325 | |
| Mean diameter (nm) | <194.8 nm | 13.7 (6.5–28.3) | Reference | Reference | ||
| >194.8 nm | 8.5 (3.8–13.8) | 1.81 (1.06–3.10) | 0.030 | 0.56 (0.28–1.12) | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badovinac, D.; Goričar, K.; Lavrin, T.; Zavrtanik, H.; Dolžan, V.; Lenassi, M.; Tomažič, A. Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study. Cancers 2023, 15, 605. https://doi.org/10.3390/cancers15030605
Badovinac D, Goričar K, Lavrin T, Zavrtanik H, Dolžan V, Lenassi M, Tomažič A. Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study. Cancers. 2023; 15(3):605. https://doi.org/10.3390/cancers15030605
Chicago/Turabian StyleBadovinac, David, Katja Goričar, Teja Lavrin, Hana Zavrtanik, Vita Dolžan, Metka Lenassi, and Aleš Tomažič. 2023. "Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study" Cancers 15, no. 3: 605. https://doi.org/10.3390/cancers15030605
APA StyleBadovinac, D., Goričar, K., Lavrin, T., Zavrtanik, H., Dolžan, V., Lenassi, M., & Tomažič, A. (2023). Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study. Cancers, 15(3), 605. https://doi.org/10.3390/cancers15030605