Outcomes Following Autologous Fat Grafting in Patients with Sequelae of Head and Neck Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
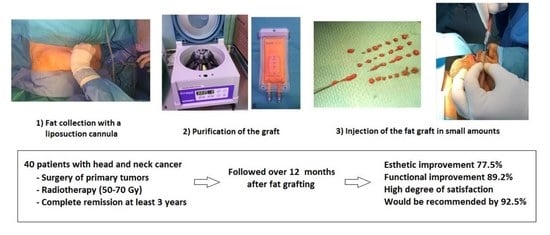
2.1. Design and Study Population
2.2. Fat Grafting and Surgical Procedure
2.3. Evaluation and Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Kang, R.; Levine, A.; Maghami, E. The new face of head and neck cancer: The HPV epidemic. Oncology 2015, 29, 616–626. [Google Scholar] [PubMed]
- Chen, M.M.; Roman, S.A.; Yarbrough, W.G.; Burtness, B.A.; Sosa, J.A.; Judson, B.L. Trends and variations in the use of adjuvant therapy for patients with head and neck cancer. Cancer 2014, 120, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Spadazzi, C.; Calpona, S.; De Rosa, F.; Usai, A.; De Vita, A.; Liverani, C.; Cocchi, C.; Vanni, S.; Calabrese, C.; et al. Precision medicine in head and neck cancers: Genomic and preclinical approaches. J. Pers. Med. 2022, 12, 854. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113 (Suppl. S7), 1911–1932. [Google Scholar] [CrossRef]
- Stepnick, D.; Gilpin, D. Head and neck cancer: An overview. Semin. Plast. Surg. 2010, 24, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Wehage, I.C.; Fansa, H. Complex reconstructions in head and neck cancer surgery: Decision making. Head Neck Oncol. 2011, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Hanasono, M.M. Reconstructive surgery for head and neck cancer patients. Adv. Med. 2014, 2014, 795483. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Dejaco, D.; Steinbichler, T.B.; Lettenbichler-Haug, A.; Anegg, M.; Ganswindt, M.; Ganswindt, U.; Gamerith, G.; Riedl, D. Functional outcomes in head and neck cancer patients. Cancers 2022, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
- Zielins, E.R.; Brett, E.A.; Longaker, M.T.; Wan, D.C. Autologous fat grafting: The science behind the surgery. Aesthet. Surg. J. 2016, 36, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Facial recontouring with lipostructure. Clin. Plast Surg. 1997, 24, 347–367. [Google Scholar] [CrossRef]
- Paolini, G.; Amoroso, M.; Longo, B.; Sorotos, M.; Karypidis, D.; Santanelli di Pompeo, F. Simplified lipostructure: A technical note. Aesthet. Plast. Surg. 2014, 38, 78–82. [Google Scholar] [CrossRef]
- Burnouf, M.; Buffet, M.; Schwarzinger, M.; Roman, P.; Bui, P.; Prévot, M.; Deleuze, J.; Morini, J.P.; Franck, N.; Gorin, I.; et al. Evaluation of Coleman lipostructure for treatment of facial lipoatrophy in patients with human immunodeficiency virus and parameters associated with the efficiency of this technique. Arch. Dermatol. 2005, 141, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Drochioi, C.I.; Sulea, D.; Timofte, D.; Mocanu, V.; Popescu, E.; Costan, V.V. Autologous fat grafting for craniofacial reconstruction in oncologic patients. Medicina 2019, 55, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez Santamaría, J.; Masiá Gridilla, J.; Pamias Romero, J.; Giralt López-de-Sagredo, J.; Bescós Atín, M.S. Fat grafting is a feasible technique for the sequelae of head and neck cancer treatment. J. Craniomaxillofac. Surg. 2017, 45, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.J.; Hanson, S.E.; Nguyen, A.T.; Skoracki, R.J.; Hanasono, M.M. Outcomes following autologous fat grafting for oncologic head and neck reconstruction. Plast. Reconstr. Surg. 2018, 142, 771–780. [Google Scholar] [CrossRef]
- Ducic, Y.; Pontius, A.T.; Smith, J.E. Lipotransfer as an adjunct in head and neck reconstruction. Laryngoscope 2003, 113, 1600–1604. [Google Scholar] [CrossRef] [Green Version]
- Krastev, T.K.; Beugels, J.; Hommes, J.; Piatkowski, A.; Mathijssen, I.; van der Hulst, R. Efficacy and safety of autologous fat transfer in facial reconstructive surgery: A systematic review and meta-analysis. JAMA Facial Plast. Surg. 2018, 20, 351–360. [Google Scholar] [CrossRef]
- Phulpin, B.; Gangloff, P.; Tran, N.; Bravetti, P.; Merlin, J.L.; Dolivet, G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast. Reconstr. Surg. 2009, 123, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural fat grafting. Aesthet. Surg. J. 1998, 18, 386–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojallal, A.; Foyatier, J.L. Historical review of the use of adipose tissue transfer in plastic and reconstructive surgery. Ann. Chir Plast Esthet. 2004, 49, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg. 2006, 118 (Suppl. 3), 108S–120S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitagliano, T.; Curto, L.S.; Greto Ciriaco, A.; Gareri, P.; Ribuffo, D.; Greco, M. Two-thirds lip defects: A new combined reconstructive technique for patients with epithelial cancer. J. Craniofac. Surg. 2016, 27, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Yu, J.C.; Arm, D.M.; Fraser, J.K. Comparison of three different fat graft preparation methods: Gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast. Reconstr. Surg. 2013, 131, 873–880. [Google Scholar] [CrossRef]
- Griffin, M.F.; Drago, J.; Almadori, A.; Kalavrezos, N.; Butler, P.E. Evaluation of the efficacy of lipotransfer to manage radiation-induced fibrosis and volume defects in head and neck oncology. Head Neck 2019, 41, 3647–3655. [Google Scholar] [CrossRef]
- Hörl, H.W.; Feller, A.M.; Biemer, E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann. Plast. Surg. 1991, 26, 248–258. [Google Scholar] [CrossRef]
- Meier, J.D.; Glasgold, R.A.; Glasgold, M.J. Autologous fat grafting: Long-term evidence of its efficacy in midfacial rejuvenation. Arch. Facial Plast. Surg. 2009, 11, 24–28. [Google Scholar] [CrossRef]





| Patient | Histology /Location | Surgery | Chemotherapy | RT Gy | Reconstruction Type | Injection Site | Volume mL | Length of Surgery min | Anesthesia | Esthetic Score Preoperative/ Postoperative | Functional Score Preoperative/ Postoperative |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ACC/parotid | PT + MD | No | 60 | Not performed | Laterocervical and parotid area | 7.5 | 50 | General | 4/3 | 3/2 |
| 2 | DFSP/malar | TEOM | No | 60 | Mustarde cheek flap | Hemifacial | 23 | 45 | General | 3/2 | 3/2 |
| 3 | SCC/tongue | MD + ND | Yes | 70 | Microsurgical fibula flap | Laterocervical and paramandibular | 28 | 65 | General | 4/4 | 4/3 |
| 4 | SCC/gums | MD + ND | Yes | 60 | Not performed | Laterocervical and paramandibular | 20 | 120 | General | 4/2 | 2/1 |
| 5 | SCC/gums | MD + ND | No | 66 | Microsurgical fibula flap | Submaxillary, lower lip, nasolabial and submental fold | 24 | 64 | General | 3/2 | 3/2 |
| 6 | SCC/gums, mouth floor | MD + ND bilateral | Yes | 70 | Fibula flap + anterolateral thigh flap | Laterocervical and paramandibular | 19 | 90 | General | 4/4 | 4/3 |
| 7 | SCC/gums | MD + ND | Yes | 50 | Not performed | Laterocervical and paramandibular | 24 | 115 | General | 4/3 | 3/2 |
| 8 | SCC/jugal mucosa | TEOM | Yes | 60 | Local flap | Jugal region, nasolabial, submental. Laterocervical, tracheocervical | 20 | 100 | General | 3/2 | 4/2 |
| 9 | SCC/gums | TEOM + MD + ND | Yes | 60 | Microsurgical fibula flap | Laterocervical and paramandibular | 37 | 135 | General | 4/4 | 3/2 |
| 10 | SCC/gums | MD + ND | Yes | 70 | Fibula flap | Laterocervical and paramandibular | 42 | 120 | General | 3/3 | 3/3 |
| 11 | SCC/tongue, mouth floor | TEOM + MD | No | 70 | Fibula flap + anterolateral thigh flap | Hemifacial and cervical | 20 | 80 | Sedation local | 4/3 | 3/2 |
| 12 | SCC/retromolar trigone | MD + ND | No | 70 | Microsurgical fibula flap | Hemifacial and cervical | 27 | 180 | General | 4/3 | 3/2 |
| 13 | SCC/jugal mucosa | TEOM + ND | No | 60 | Radial flap | Laterocervical, paramandibular, jugal | 23 | 110 | General | 3/2 | 2/1 |
| 14 | SCC/mouth floor | MD + ND | Yes | 70 | Microsurgical fibula flap | Laterocervical, paramandibular, tracheal | 70 | 100 | General | 4/3 | 4/2 |
| 15 | Myoepithelial carcinoma/ minor salivary gland, maxillary | Maxillectomy | No | 60 | Temporal muscle flap | Temporal | 13 | 150 | General | 3/1 | 0/0 |
| 16 | Myoepithelial carcinoma/parotid gland | Radical Parotidectomy + ND | Yes | 66 | Not performed | Laterocervical and parotid region | 8.5 | 58 | General | 3/2 | 2/1 |
| 17 | SCC/gums | MD + ND | Yes | 70 | Microsurgical fibula flap | Hemifacial and cervical | 15 | 110 | General | 3/2 | 3/1 |
| 18 | SCC/gums | Maxillectomy + ND | Yes | 64 | Microsurgical fibula flap | Hemifacial and cervical | 23 | 70 | General | 3/2 | 3/2 |
| 19 | Adenocarcinoma parotid gland | Total parotidectomy | No | 50 | Not performed | Hemifacial | 13 | 87 | General | 2/0 | 2/0 |
| 20 | SCC/retromolar trigone | MD + ND | Yes | 70 | Not performed | Nasolabial fold, upper and lower lip, jugal and laterocervical | 120 | 170 | General | 4/3 | 4/2 |
| 21 | SCC/cervical unknown origin | ND | No | 60 | Not performed | Laterocervical | 15 | 52 | Sedation local | 3/4 | 4/4 |
| 22 | SCC/tongue, mouth floor | Glossectomy + MD + ND | No | 66 | Microsurgical fibula flap | Upper and lower lips, paramandibular, superior laterocervical, bilateral submandibular, bilateral nasolabial folds | 20 | 117 | General | 4/2 | 3/1 |
| 23 | SCC/mouth floor | MD + ND bilateral | No | 70 | Microsurgical fibula flap | Lower lip, paramandibular, laterocervical | 12 | 97 | General | 4/2 | 3/2 |
| 24 | Ductal carcinoma/parotid gland | Superficial parotidectomy | No | 60 | Not performed | Laterocervical and parotid region | 15 | 71 | General | 2/1 | 2/1 |
| 25 | SCC/retromolar trigone | MD + ND | Yes | 70 | Microsurgical fibula flap | Paramandibular, submaxillary, upper and lower lip | 23 | 63 | General | 4/3 | 4/2 |
| 26 | SCC/jugal mucosa | TEOM + ND | Yes | 66 | Local flap | Laterocervical, paramandibular, jugal | 23 | 79 | General | 3/2 | 4/2 |
| 27 | SCC/mandibular intraosseous | MD + ND bilateral | Yes | 63 | Microsurgical fibula flap | Paramandibular, laterocervical, nasolabial fold, lower lip | 23 | 103 | General | 4/2 | 4/2 |
| 28 | Undifferentiated parotid carcinoma | Total parotidectomy | No | 60 | Not performed | Paramandibular and parotid region | 25 | 83 | General | 4/2 | 3/1 |
| 29 | SCC/cervical unknown origin | ND | Yes | 60 | Not performed | Laterocervical | 14 | 47 | General | 3/2 | 4/3 |
| 30 | SCC/tongue | Glossectomy + ND | Yes | 54 | Nor performed | Laterocervical, lingual | 18 | 115 | General | 3/3 | 4/4 |
| 31 | ACC/minor salivary gland maxillary | Maxillectomy | No | 66 | Temporal muscle flap | Malar bilateral, left nasolabial fold, upper lip, left jugal | 20 | 47 | General | 3/2 | 0/0 |
| 32 | SCC/gums | MD + ND | Yes | 70 | Pectoral flap | Paramandibular and jugal | 10 | 48 | General | 4/2 | 2/1 |
| 33 | SCC/retromolar trigone | MD + ND | Yes | 70 | Pectoral flap + fibula flap | Hemifacial, cervical, labial, tracheal | 15 | 114 | General | 4/2 | 3/2 |
| 34 | SCC/lip | TEOM + ND | No | 55 | Anterolateral thigh flap | Jugal and labial | 8 | 54 | Sedation local | 2/2 | 0/0 |
| 35 | ACC/oropharynx-tongue | Glossectomy + ND | No | 66 | Anterolateral thigh flap | Laterocervical | 20 | 104 | General | 3/3 | 3/3 |
| 36 | SCC/gums, mouth floor | MD + ND | Yes | 69 | Not performed | Right laterocervical, paramandibular | 15 | 55 | General | 4/4 | 4/2 |
| 37 | Angiofibroma/nasal | TEOM | No | 50 | Not performed | Temporal | 20 | 95 | General | 3/2 | 1/0 |
| 38 | SCC/mandibular symphysis | MD + ND bilateral | Yes | 70 | Microsurgical fibula flap | Laterocervical and submental | 10 | 48 | General | 4/3 | 3/2 |
| 39 | Osteosarcoma mandibular | MD + maxillectomy + ND | Yes | 60 | Anterolateral thigh flap | Hemifacial and cervical | 10 | 70 | General | 4/3 | 3/2 |
| 40 | ACC/upper maxilla | Maxillectomy + ND | Yes | 72 | Temporal muscle flap + microsurgical fibula flap | Hemifacial, cervical and temporal | 27 | 76 | General | 3/2 | 3/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masià-Gridilla, J.; Gutiérrez-Santamaría, J.; Álvarez-Sáez, I.; Pamias-Romero, J.; Saez-Barba, M.; Bescós-Atin, C. Outcomes Following Autologous Fat Grafting in Patients with Sequelae of Head and Neck Cancer Treatment. Cancers 2023, 15, 800. https://doi.org/10.3390/cancers15030800
Masià-Gridilla J, Gutiérrez-Santamaría J, Álvarez-Sáez I, Pamias-Romero J, Saez-Barba M, Bescós-Atin C. Outcomes Following Autologous Fat Grafting in Patients with Sequelae of Head and Neck Cancer Treatment. Cancers. 2023; 15(3):800. https://doi.org/10.3390/cancers15030800
Chicago/Turabian StyleMasià-Gridilla, Jorge, Javier Gutiérrez-Santamaría, Iago Álvarez-Sáez, Jorge Pamias-Romero, Manel Saez-Barba, and Coro Bescós-Atin. 2023. "Outcomes Following Autologous Fat Grafting in Patients with Sequelae of Head and Neck Cancer Treatment" Cancers 15, no. 3: 800. https://doi.org/10.3390/cancers15030800
APA StyleMasià-Gridilla, J., Gutiérrez-Santamaría, J., Álvarez-Sáez, I., Pamias-Romero, J., Saez-Barba, M., & Bescós-Atin, C. (2023). Outcomes Following Autologous Fat Grafting in Patients with Sequelae of Head and Neck Cancer Treatment. Cancers, 15(3), 800. https://doi.org/10.3390/cancers15030800