Tumour Cell Seeding to Lymph Nodes from In Situ Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
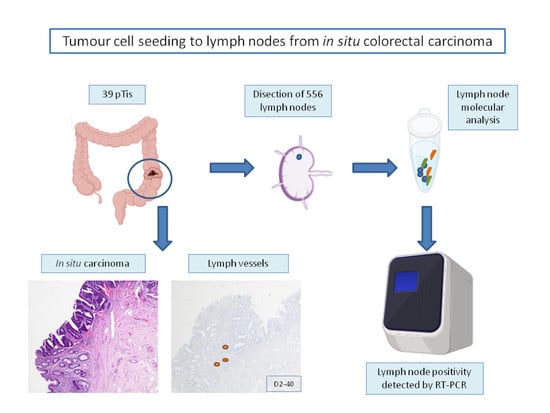
2. Materials and Methods
2.1. Patients and Tumours
2.2. Ethical Considerations
2.3. Immunohistochemistry Staining
2.4. Lymphatic Vessel Assessment
2.5. Intratumour Budding Evaluation
2.6. Lymph Node Dissection and Analysis
2.7. One Step Nucleic Acid Amplification (OSNA) Assay
2.8. Lymph Node Staging
2.9. Statistical Analysis
3. Results
3.1. Study Sample
3.2. The Normal Colonic Mucosa Is Devoid of Lymphatic Vessels
3.3. Presence of Lymphatic Vessels in the Mucosa of In Situ CRC
3.4. Intratumoural Tumour Budding in pTis
3.5. Detection of Tumour Burden in Regional LNs with the OSNA Assay
3.6. Patient’s Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fogt, F.; Zimmerman, R.L.; Ross, H.M.; Daly, T.; Gausas, R.E. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol. Rep. 2004, 11, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, C.M.; Kaye, G.I.; Lane, N. Distribution of Human Colonic Lymphatics in Normal, Hyperplastic, and Adenomatous Tissue: Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterology 1973, 64, 51–66. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Bork, U.; Motschall, E.; Thorlund, K.; Büchler, M.W.; Koch, M.; Weitz, J. Molecular detection of tumour cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 60–70. [Google Scholar] [CrossRef]
- Sacchi, G.; Weber, E.; Aglianó, M.; Lorenzoni, P.; Rossi, A.; Caruso, A.M.; Vernillo, R.; Gerli, R.; Lorenzi, M. Lymphatic vessels in colorectal cancer and their relation with inflammatory infiltrate. Dis. Colon Rectum 2003, 46, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kenney, B.C.; Jain, D. Identification of Lymphatics within the Colonic Lamina Propria in Inflammation and Neoplasia Using the Monoclonal Antibody D2-40. Yale J. Biol. Med. 2008, 81, 103–113. [Google Scholar] [PubMed]
- Tomita, T. Immunocytochemical localization of lymphatic and venous vessels in colonic polyps and adenomas. Dig. Dis. Sci. 2008, 53, 1880–1885. [Google Scholar] [CrossRef]
- Peg, V.; Espinosa-Bravo, M.; Vieites, B.; Vilardell, F.; Antúnez, J.R.; de Salas, M.S.; Delgado-Sánchez, J.J.; Pinto, W.; Gozalbo, F.; Petit, A.; et al. Intraoperative molecular analysis of total tumour load in sentinel lymph node: A new predictor of axillary status in early breast cancer patients. Breast Cancer Res. Treat. 2013, 139, 87–93. [Google Scholar] [CrossRef]
- Yamamoto, N.; Daito, M.; Hiyama, K.; Ding, J.; Nakabayashi, K.; Otomo, Y.; Tsujimoto, M.; Matsuura, N.; Kato, Y. An optimal mRNA marker for OSNA (One-step nucleic acid amplification) based lymph node metastasis detection in colorectal cancer patients. Jpn. J. Clin. Oncol. 2013, 43, 264–270. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sekimoto, M.; Oya, M.; Yamamoto, N.; Konishi, F.; Sasaki, J.; Yamada, S.; Taniyama, K.; Tominaga, H.; Tsujimoto, M.; et al. OSNA-based novel molecular testing for lymph node metastases in colorectal cancer patients: Results from a multicenter clinical performance study in Japan. Ann. Surg. Oncol. 2011, 18, 1891–1898. [Google Scholar] [CrossRef]
- Itabashi, M.; Yamamoto, H.; Tomita, N.; Inomata, M.; Murata, K.; Hayashi, S.; Miyake, Y.; Igarashi, S.; Kato, T.; Noura, S.; et al. Lymph Node Positivity in One-Step Nucleic Acid Amplification is a Prognostic Factor for Postoperative Cancer Recurrence in Patients with Stage II Colorectal Cancer: A Prospective, Multicenter Study. Ann. Surg. Oncol. 2020, 27, 1077–1083. [Google Scholar] [CrossRef]
- Archilla, I.; Díaz-Mercedes, S.; Aguirre, J.J.; Tarragona, J.; Machado, I.; Rodrigo, M.T.; Lopez-Prades, S.; Gorostiaga, I.; Landolfi, S.; Alén, B.O.; et al. Lymph Node Tumour Burden Correlates With Tumour Budding and Poorly Differentiated Clusters: A New Prognostic Factor in Colorectal Carcinoma? Clin. Transl. Gastroenterol. 2021, 12, e00303. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Horiuchi, H.; Kurata, A.; Kikuchi, H.; Okuyama, R.; Usui, G.; Masuda, Y.; Kuroda, M.; Inoue, S.; Furushima, K.; et al. Intramucosal colorectal carcinoma with lymphovascular invasion: Clinicopathological characteristics of nine cases. Histopathology 2019, 74, 1055–1066. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Martin, J.C.; Boschetti, G.; Chang, C.; Ungaro, R.; Giri, M.; Chuang, L.S.; Nayar, S.; Greenstein, A.; Dubinsky, M.; Walker, L.; et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. bioRxiv 2018, 178, 1493–1508. [Google Scholar] [CrossRef]
- Duff, S.E.; Jeziorska, M.; Kumar, S.; Haboubi, N.; Sherlock, D.; O’dwyer, S.T.; Jayson, G.C. Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Color. Dis. 2007, 9, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Zhou, Y.J.; Bin, D.; Qiangchen; Wang, S.Y. Clinical significance of detecting lymphatic and blood vessel invasion in stage II colon cancer using markers D2-40 and CD34 in combination. Asian Pac. J. Cancer Prev. 2014, 15, 1363–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumour budding in colorectal cancer based on the International Tumour Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlobec, I.; Bächli, M.; Galuppini, F.; Berger, M.D.; Dawson, H.E.; Nagtegaal, I.D.; Lugli, A. Refining the ITBCC tumour budding scoring system with a “zero-budding” category in colorectal cancer. Virchows Arch. 2021, 479, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Montironi, C.; Aldecoa, I.; Fernandez, E.; Bombi, J.A.; Jimeno, M.; Balaguer, F.; Pellise, M.; Castells, A.; Cuatrecasas, M. Lymph node pooling: A feasible and efficient method of lymph node molecular staging in colorectal carcinoma. J. Transl. Med. 2017, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, M.; Nakabayashi, K.; Yoshidome, K.; Kaneko, T.; Iwase, T.; Akiyama, F.; Kato, Y.; Tsuda, H.; Ueda, S.; Sato, K.; et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin. Cancer Res. 2007, 13, 4807–4816. [Google Scholar] [CrossRef] [Green Version]
- Bracey, T.; Mathew, J. Metastatic intramucosal colorectal adenocarcinoma: A case to support review of current concepts (and staging) of early colorectal cancer. Histopathology 2015, 66, 906–907. [Google Scholar] [CrossRef]
- Lan, Y.T.; Yang, S.H.; Li, A.F.Y.; Lin, J.K. Conflicting finding on intramucosal colon cancers based on national survival outcomes data. J. Clin. Oncol. 2010, 28, 2433. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; Fenton, H.; Burkart, A.L.; Sheridan, T.; Abu-Alfa, A.K.; Montgomery, E.A. Poorly differentiated colorectal carcinoma with invasion restricted to lamina propria (intramucosal carcinoma): A follow-up study of 15 cases. Am. J. Surg. Pathol. 2007, 31, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Shia, J.; Klimstra, D.S. Intramucosal poorly differentiated colorectal carcinoma: Can it be managed conservatively? Am. J. Surg. Pathol. 2008, 32, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, I.; Montironi, C.; Planell, N.; Pellise, M.; Fernandez-Esparrach, G.; Gines, A.; Delgado, S.; Momblan, D.; Moreira, L.; Lopez-Ceron, M.; et al. Endoscopic tattooing of early colon carcinoma enhances detection of lymph nodes most prone to harbor tumour burden. Surg. Endosc. Other Interv. Tech. 2016, 31, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyoshi, Y.; Akiyoshi, T.; Fukunaga, Y. The advantage of one-step nucleic acid amplification for the diagnosis of lymph node metastasis in colorectal cancer patients. Ann. Gastroenterol. Surg. 2020, 5, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, N.C.A.; Backes, Y.; Snijders, H.S.; Bastiaannet, E.; Liefers, G.J.; Moons, L.M.G.; Van De Velde, C.J.H.; Peeters, K.C.M.J. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open 2018, 3, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.Y.; Yun, H.R.; Kim, S.H.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; Chun, H.K.; Cho, Y.B. Features of late recurrence following transanal local excision for early rectal cancer. Dis. Colon Rectum 2015, 58, 1041–1047. [Google Scholar] [CrossRef]


| Category | No (Total) | No (%) |
|---|---|---|
| Gender | ||
| Male | 23 | 59 |
| Female | 16 | 41 |
| Age (year) | ||
| <65 | 17 | 44 |
| ≥65 | 22 | 56 |
| Tumour size (cm) | ||
| <2 | 23 | 59 |
| ≥2 | 16 | 41 |
| Tumour location | ||
| Right colon | 31 | 79 |
| Left colon and rectum | 8 | 21 |
| Lymphatic vessels | ||
| Low LV | 25 | 64 |
| High LV | 14 | 36 |
| Tumour budding | ||
| TB-negative (Bd0) | 30 | 77 |
| TB-positive (Bd1, Bd2) | 9 | 23 |
| OSNA | ||
| Positive | 11 | 28 |
| Negative | 28 | 72 |
| LN | ||
| Fresh | 556 | 86 |
| Formol | 92 | 14 |
| Category | High-LV (Number) | Low-LV (Number) | p |
|---|---|---|---|
| Gender | |||
| Male | 2 | 21 | 0.538 |
| Female | 3 | 13 | |
| Age (year) | |||
| <65 | 1 | 16 | 0.428 |
| ≥65 | 3 | 19 | |
| Tumour size (cm) | |||
| <2 | 3 | 20 | 0.491 |
| ≥2 | 1 | 15 | |
| Tumour location | |||
| Right colon | 3 | 28 | 0.176 |
| Left colon and rectum | 2 | 6 | |
| Tumour budding | |||
| TB-negative (Bd0) | 3 | 27 | 0.923 |
| TB-positive (Bd1, Bd2) | 1 | 8 |
| Category | OSNA + (Number) | OSNA – (Number) | p |
|---|---|---|---|
| Gender | |||
| Male | 7 | 16 | 0.711 |
| Female | 4 | 12 | |
| Age (years) | |||
| Mean | 65.72 | 69.78 | |
| <65 | 6 | 11 | 0.387 |
| ≥65 | 17 | 5 | |
| Tumour size (cm) | |||
| Mean | 2.8 | 1.9 | |
| <2 | 5 | 18 | 0.293 |
| ≥2 | 6 | 10 | |
| Tumour location | |||
| Right colon | 9 | 26 | 0.307 |
| Left colon and rectum | 2 | 2 | |
| Lymphatic vessels | |||
| Mean | 2.8 | 2.3 | |
| Low LV | 9 | 25 | 0.357 |
| High LV | 2 | 3 | |
| Tumour budding | |||
| TB-negative (Bd0) | 7 | 23 | 0.217 |
| TB-positive (Bd1, Bd2) | 4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo-Calvo, M.T.; Saez de Gordoa, K.; Lopez-Prades, S.; Archilla, I.; Diaz, A.; Berrios, M.; Camps, J.; Musulen, E.; Cuatrecasas, M. Tumour Cell Seeding to Lymph Nodes from In Situ Colorectal Cancer. Cancers 2023, 15, 842. https://doi.org/10.3390/cancers15030842
Rodrigo-Calvo MT, Saez de Gordoa K, Lopez-Prades S, Archilla I, Diaz A, Berrios M, Camps J, Musulen E, Cuatrecasas M. Tumour Cell Seeding to Lymph Nodes from In Situ Colorectal Cancer. Cancers. 2023; 15(3):842. https://doi.org/10.3390/cancers15030842
Chicago/Turabian StyleRodrigo-Calvo, Maria Teresa, Karmele Saez de Gordoa, Sandra Lopez-Prades, Ivan Archilla, Alba Diaz, Mario Berrios, Jordi Camps, Eva Musulen, and Miriam Cuatrecasas. 2023. "Tumour Cell Seeding to Lymph Nodes from In Situ Colorectal Cancer" Cancers 15, no. 3: 842. https://doi.org/10.3390/cancers15030842
APA StyleRodrigo-Calvo, M. T., Saez de Gordoa, K., Lopez-Prades, S., Archilla, I., Diaz, A., Berrios, M., Camps, J., Musulen, E., & Cuatrecasas, M. (2023). Tumour Cell Seeding to Lymph Nodes from In Situ Colorectal Cancer. Cancers, 15(3), 842. https://doi.org/10.3390/cancers15030842