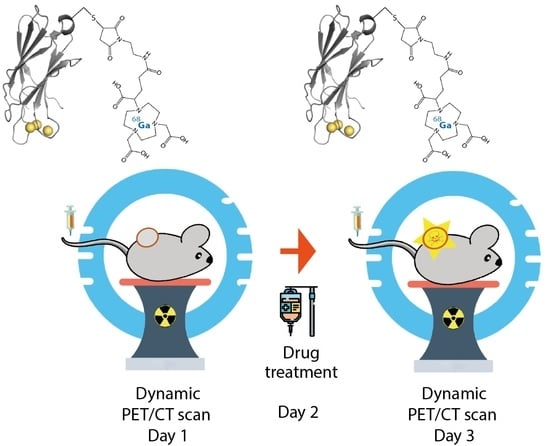
Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of 68Ga-C2Am
2.2. Cell Culture
2.3. Dead Cell Labeling with 68Ga-C2Am
2.4. Animal Studies
2.5. Dynamic PET/CT Imaging of Tumor Cell Death Using 68Ga-C2Am
3. Results
3.1. Radiolabeling of C2Am Using Gallium-68 Chloride
3.2. Cell Binding Assays Using 68Ga-C2Am
3.3. Stability of 68Ga-C2Am
3.4. Biodistribution of 68Ga-C2Am
3.5. PET-CT Imaging of Tumor Cell Death In Vivo Using 68Ga-C2Am
3.6. Correlation of Tumor Contrast with the Levels of Cell Death Determined Histologically
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brindle, K.M. New Approaches for Imaging Tumour Responses to Treatment. Nat. Rev. Cancer 2008, 8, 94–107. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Sheikhbahaei, S.; Mena, E.; Yanamadala, A.; Reddy, S.; Solnes, L.B.; Wachsmann, J.; Subramaniam, R.M. The Value of FDG PET/CT in Treatment Response Assessment, Follow-Up, and Surveillance of Lung Cancer. Am. J. Roentgenol. 2017, 208, 420–433. [Google Scholar] [CrossRef]
- Neves, A.A.; Brindle, K.M. Imaging Cell Death. J. Nucl. Med. 2014, 55, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Jiang, H.; Liu, Y.; Zhang, H.; Tian, M. Radionuclide Imaging of Apoptosis for Clinical Application. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1345–1359. [Google Scholar] [CrossRef]
- van de Wiele, C.; Ustmert, S.; de Spiegeleer, B.; de Jonghe, P.J.; Sathekge, M.; Alex, M. Apoptosis Imaging in Oncology by Means of Positron Emission Tomography: A Review. Int. J. Mol. Sci. 2021, 22, 2753. [Google Scholar] [CrossRef]
- Elvas, F.; Stroobants, S.; Wyffels, L. Phosphatidylethanolamine Targeting for Cell Death Imaging in Early Treatment Response Evaluation and Disease Diagnosis. Apoptosis 2017, 22, 971–987. [Google Scholar] [CrossRef]
- Dubash, S.R.; Merchant, S.; Heinzmann, K.; Mauri, F.; Lavdas, I.; Inglese, M.; Kozlowski, K.; Rama, N.; Masrour, N.; Steel, J.F.; et al. Clinical Translation of [18F]ICMT-11 for Measuring Chemotherapy-Induced Caspase 3/7 Activation in Breast and Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2285–2299. [Google Scholar] [CrossRef] [Green Version]
- Jouberton, E.; Schmitt, S.; Maisonial-Besset, A.; Chautard, E.; Penault-Llorca, F.; Cachin, F. Interest and Limits of [18F]ML-10 PET Imaging for Early Detection of Response to Conventional Chemotherapy. Front. Oncol. 2021, 11, 789769. [Google Scholar] [CrossRef]
- Park, D.; Don, A.S.; Massamiri, T.; Karwa, A.; Warner, B.; MacDonald, J.; Hemenway, C.; Naik, A.; Kuan, K.-T.; Dilda, P.J.; et al. Noninvasive Imaging of Cell Death Using an Hsp90 Ligand. J. Am. Chem. Soc. 2011, 133, 2832–2835. [Google Scholar] [CrossRef] [Green Version]
- Ho Shon, I.; Hennessy, T.; Guille, J.; Gotsbacher, M.P.; Lay, A.J.; McBride, B.; Codd, R.; Hogg, P.J. A First-in-Human Study of [68Ga]Ga-CDI: A Positron Emitting Radiopharmaceutical for Imaging Tumour Cell Death. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4037–4047. [Google Scholar] [CrossRef]
- Clark, M.R. Flippin’ Lipids. Nat. Immunol. 2011, 12, 373–375. [Google Scholar] [CrossRef]
- Elvas, F.; Vangestel, C.; Pak, K.; Vermeulen, P.; Gray, B.; Stroobants, S.; Staelens, S.; Wyffels, L. Early Prediction of Tumor Response to Treatment: Preclinical Validation of 99mTc-Duramycin. J. Nucl. Med. 2016, 57, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Hu, K.; Tang, G.; Liang, X.; Du, K.; Nie, D.; Jiang, S.; Zang, L. Positron Emission Tomography Imaging of Cell Death with [18F]FPDuramycin. Apoptosis 2014, 19, 841–850. [Google Scholar] [CrossRef]
- Rix, A.; Drude, N.I.; Mrugalla, A.; Baskaya, F.; Pak, K.Y.; Gray, B.; Kaiser, H.-J.; Tolba, R.H.; Fiegle, E.; Lederle, W.; et al. Assessment of Chemotherapy-Induced Organ Damage with Ga-68 Labeled Duramycin. Mol. Imaging Biol. 2019, 22, 623–633. [Google Scholar] [CrossRef]
- Tait, J.F.; Gibson, D. Phospholipid Binding of Annexin V: Effects of Calcium and Membrane Phosphatidylserine Content. Arch. Biochem. Biophys. 1992, 298, 187–191. [Google Scholar] [CrossRef]
- Vangestel, C.; Peeters, M.; Mees, G.; Oltenfreiter, R.; Boersma, H.H.; Elsinga, P.H.; Reutelingsperger, C.; van Damme, N.; de Spiegeleer, B.; van de Wiele, C. In Vivo Imaging of Apoptosis in Oncology: An Update. Mol. Imaging 2011, 10, 340–358. [Google Scholar] [CrossRef]
- Belhocine, T.; Steinmetz, N.; Hustinx, R.; Bartsch, P.; Jerusalem, G.; Seidel, L.; Rigo, P.; Green, A. Increased Uptake of the Apoptosis-Imaging Agent 99mTc Recombinant Human Annexin V in Human Tumors after One Course of Chemotherapy as a Predictor of Tumor Response and Patient Prognosis1. Clin. Cancer Res. 2002, 8, 2766–2774. [Google Scholar]
- Bauwens, M.; de Saint-Hubert, M.; Devos, E.; Deckers, N.; Reutelingsperger, C.; Mortelmans, L.; Himmelreich, U.; Mottaghy, F.M.; Verbruggen, A. Site-Specific 68Ga-Labeled Annexin A5 as a PET Imaging Agent for Apoptosis. Nucl. Med. Biol. 2011, 38, 381–392. [Google Scholar] [CrossRef]
- Murakami, Y.; Takamatsu, H.; Taki, J.; Tatsumi, M.; Noda, A.; Ichise, R.; Tait, J.F.; Nishimura, S. 18F-Labelled Annexin V: A PET Tracer for Apoptosis Imaging. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 469–474. [Google Scholar] [CrossRef]
- Li, X.; Link, J.M.; Stekhova, S.; Yagle, K.J.; Smith, C.; Krohn, K.A.; Tait, J.F. Site-Specific Labeling of Annexin V with F-18 for Apoptosis Imaging. Bioconjug. Chem. 2008, 19, 1684–1688. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Jiang, Q.; Hu, M.; Tan, C.; Yu, H.; Hua, Z. Preliminary Biological Evaluation of 18F-FBEM-Cys-Annexin V a Novel Apoptosis Imaging Agent. Molecules 2015, 20, 4902–4914. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Jiang, Q.; Hu, M.; Tan, C.; Yu, H.; Hua, Z. Preliminary Biological Evaluation of 18F-AlF-NOTA-MAL-Cys-Annexin V as a Novel Apoptosis Imaging Agent. Oncotarget 2017, 8, 51086–51095. [Google Scholar] [CrossRef] [Green Version]
- Alam, I.S.; Neves, A.A.; Witney, T.H.; Boren, J.; Brindle, K.M. Comparison of the C2A Domain of Synaptotagmin-I and Annexin-V as Probes for Detecting Cell Death. Bioconjug. Chem. 2010, 21, 884–891. [Google Scholar] [CrossRef]
- Zhao, M.; Beauregard, D.A.; Loizou, L.; Davletov, B.; Brindle, K.M. Non-Invasive Detection of Apoptosis Using Magnetic Resonance Imaging and a Targeted Contrast Agent. Nat. Med. 2001, 7, 1241–1244. [Google Scholar] [CrossRef]
- Krishnan, A.S.; Neves, A.A.; de Backer, M.M.; Hu, D.-E.; Davletov, B.; Kettunen, M.I.; Brindle, K.M. Detection of Cell Death in Tumors by Using MR Imaging and a Gadolinium-Based Targeted Contrast Agent. Radiology 2008, 246, 854–862. [Google Scholar] [CrossRef]
- Fang, W.; Wang, F.; Ji, S.; Zhu, X.; Meier, H.T.; Hellman, R.S.; Brindle, K.M.; Davletov, B.; Zhao, M. SPECT Imaging of Myocardial Infarction Using 99mTc-Labeled C2A Domain of Synaptotagmin I in a Porcine Ischemia–Reperfusion Model. Nucl. Med. Biol. 2007, 34, 917–923. [Google Scholar] [CrossRef]
- Neves, A.A.; Xie, B.; Fawcett, S.; Alam, I.S.; Witney, T.H.; de Backer, M.M.; Summers, J.; Hughes, W.; McGuire, S.; Soloviev, D.; et al. Rapid Imaging of Tumor Cell Death In Vivo Using the C2A Domain of Synaptotagmin-I. J. Nucl. Med. 2017, 58, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Tomaszewski, M.R.; Neves, A.A.; Ros, S.; Hu, D.-E.; McGuire, S.; Mullins, S.R.; Tice, D.; Sainson, R.C.A.; Bohndiek, S.E.; et al. Optoacoustic Detection of Early Therapy-Induced Tumor Cell Death Using a Targeted Imaging Agent. Clin. Cancer Res. 2017, 23, 6893–6903. [Google Scholar] [CrossRef] [Green Version]
- Bulat, F.; Hesse, F.; Hu, D.E.; Ros, S.; Willminton-Holmes, C.; Xie, B.; Attili, B.; Soloviev, D.; Aigbirhio, F.; Leeper, F.J.; et al. 18F-C2Am: A Targeted Imaging Agent for Detecting Tumor Cell Death In Vivo Using Positron Emission Tomography. EJNMMI Res. 2020, 10, 151. [Google Scholar] [CrossRef]
- Ducharme, J.; Goertzen, A.L.; Patterson, J.; Demeter, S. Practical Aspects of 18F-FDG PET When Receiving 18F-FDG from a Distant Supplier. J. Nucl. Med. Technol. 2009, 37, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, R.; Dash, A. Nanomaterial-Based Adsorbents: The Prospect of Developing New Generation Radionuclide Generators to Meet Future Research and Clinical Demands. J. Radioanal. Nucl. Chem. 2013, 299, 741–757. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Deppen, S.A.; Liu, E.; Blume, J.D.; Clanton, J.; Shi, C.; Jones-Jackson, L.B.; Lakhani, V.; Baum, R.P.; Berlin, J.; Smith, G.T.; et al. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Lugat, A.; Frampas, É.; Touchefeu, Y.; Mirallié, É.; Bras, M.L.; Senellart, H.; Rauscher, A.; Fleury, V.; Campion, L.; Rohmer, V.; et al. Prospective Multicentric Assessment of 68Ga-DOTANOC PET/CT in Grade 1-2 GEP-NET. Cancers 2023, 15, 513. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [Green Version]
- Hesketh, R.L.; Wang, J.; Wright, A.J.; Lewis, D.Y.; Denton, A.E.; Grenfell, R.; Miller, J.L.; Bielik, R.; Gehrung, M.; Fala, M.; et al. Magnetic Resonance Imaging Is More Sensitive Than PET for Detecting Treatment-Induced Cell Death-Dependent Changes in Glycolysis. Cancer Res. 2019, 79, 3557–3569. [Google Scholar] [CrossRef]
- Hesse, F.; Somai, V.; Kreis, F.; Bulat, F.; Wright, A.J.; Brindle, K.M. Monitoring Tumor Cell Death in Murine Tumor Models Using Deuterium Magnetic Resonance Spectroscopy and Spectroscopic Imaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2014631118. [Google Scholar] [CrossRef]
- Finnberg, N.K.; Gokare, P.; Navaraj, A.; Lang Kuhs, K.A.; Cerniglia, G.; Yagita, H.; Takeda, K.; Motoyama, N.; El-Deiry, W.S. Agonists of the TRAIL Death Receptor DR5 Sensitize Intestinal Stem Cells to Chemotherapy-Induced Cell Death and Trigger Gastrointestinal Toxicity. Cancer Res. 2016, 76, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Kiessling, V.; Cafiso, D.S.; Tamm, L.K. Partitioning of Synaptotagmin I C2 Domains between Liquid-Ordered and Liquid-Disordered Inner Leaflet Lipid Phases. Biochemistry 2011, 50, 2478–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsin, S.K.; Weiss, H.L.; Gutierrez, M.C.; Chamness, G.C.; Schiff, R.; DiGiovanna, M.P.; Wang, C.-X.; Hilsenbeck, S.G.; Osborne, C.K.; Allred, D.C.; et al. Neoadjuvant Trastuzumab Induces Apoptosis in Primary Breast Cancers. J. Clin. Oncol. 2005, 23, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Bulat, F.; Hesse, F.; Attili, B.; Solanki, C.; Mendichovszky, I.; Aigbirhio, F.; Leeper, F.; Brindle, K.; Neves, A. Research data supporting Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am; Apollo, University of Cambridge Repository: Cambridge, UK, 2023. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulat, F.; Hesse, F.; Attili, B.; Solanki, C.; Mendichovszky, I.A.; Aigbirhio, F.; Leeper, F.J.; Brindle, K.M.; Neves, A.A. Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am. Cancers 2023, 15, 1564. https://doi.org/10.3390/cancers15051564
Bulat F, Hesse F, Attili B, Solanki C, Mendichovszky IA, Aigbirhio F, Leeper FJ, Brindle KM, Neves AA. Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am. Cancers. 2023; 15(5):1564. https://doi.org/10.3390/cancers15051564
Chicago/Turabian StyleBulat, Flaviu, Friederike Hesse, Bala Attili, Chandra Solanki, Iosif A. Mendichovszky, Franklin Aigbirhio, Finian J. Leeper, Kevin M. Brindle, and André A. Neves. 2023. "Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am" Cancers 15, no. 5: 1564. https://doi.org/10.3390/cancers15051564
APA StyleBulat, F., Hesse, F., Attili, B., Solanki, C., Mendichovszky, I. A., Aigbirhio, F., Leeper, F. J., Brindle, K. M., & Neves, A. A. (2023). Preclinical PET Imaging of Tumor Cell Death following Therapy Using Gallium-68-Labeled C2Am. Cancers, 15(5), 1564. https://doi.org/10.3390/cancers15051564