Higher Age (≥60 Years) Increases the Risk for Adverse Events during Autologous Hematopoietic Stem Cell Transplantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and autoHSCT
2.2. Parameters for Analyses
2.3. AEs
2.4. Statistics
3. Results
3.1. Characteristics of Patients and Transplants
3.2. Adverse Events (AEs)
3.3. Transplantations with AEs
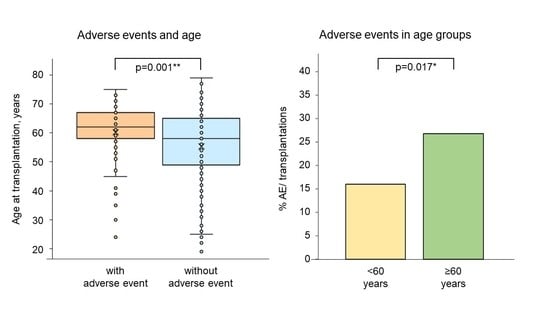
3.4. AEs and Age at Transplantation
3.5. Number of AEs per Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chabannon, C.; Kuball, J.; Bondanza, A.; Dazzi, F.; Pedrazzoli, P.; Toubert, A.; Ruggeri, A.; Fleischhauer, K.; Bonini, C. Hematopoietic stem cell transplantation in its 60s: A platform for cellular therapies. Sci. Transl. Med. 2018, 10, eaap9630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2018; ISBN 978-3-030-02277-8. [Google Scholar]
- Kanate, A.S.; Majhail, N.S.; Savani, B.N.; Bredeson, C.; Champlin, R.E.; Crawford, S.; Giralt, S.A.; LeMaistre, C.F.; Marks, D.I.; Omel, J.L.; et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: Guidelines from the American society for transplantation and cellular therapy. Biol. Blood Marrow Transplant. 2020, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- FACT-JACIE International Standards for HEMATOPOIETIC CELLULAR THERAPY Product Collection, Processing, and Administration; FACT-JACIE International Standards; Eighth Edition 8.1; Foundation for the Accreditation of Cellular Therapy (Fact): Omaha, NE, USA; Joint Accreditation Committee -isct and ebmt (JACIE): Barcelona, Spain, 2021.
- Directorate-General for Health and Food Safety. Summary of the 2020 Annual Reporting of Serious Adverse Reactions and Events for Tissues and Cells; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Donmez, A.; Tombuloglu, M.; Gungor, A.; Soyer, N.; Saydam, G.; Cagirgan, S. Clinical side effects during peripheral blood progenitor cell infusion. Transfus. Apher. Sci. 2007, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research 2019, 7, 1746. [Google Scholar] [CrossRef]
- Ikeda, K.; Ohto, H.; Okuyama, Y.; Yamada-Fujiwara, M.; Kanamori, H.; Fujiwara, S.; Muroi, K.; Mori, T.; Kasama, K.; Iseki, T.; et al. Adverse events associated with infusion of hematopoietic stem cell products: A prospective and multicenter surveillance study. Transfus. Med. Rev. 2018, 32, 186–194. [Google Scholar] [CrossRef]
- Abrahamsen, J.F.; Bakken, A.M.; Bruserud. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 2002, 42, 1573–1580. [Google Scholar] [CrossRef]
- Abrahamsen, J.F.; Rusten, L.; Bakken, A.M.; Bruserud. Better preservation of early hematopoietic progenitor cells when human peripheral blood progenitor cells are cryopreserved with 5 percent dimethylsulfoxide instead of 10 percent dimethylsulfoxide: LTC-ICs in PBPCs frozen with 5 and 10 percent DMSO. Transfusion 2004, 44, 785–789. [Google Scholar] [CrossRef]
- Morris, C.; de Wreede, L.; Scholten, M.; Brand, R.; van Biezen, A.; Sureda, A.; Dickmeiss, E.; Trneny, M.; Apperley, J.; Chiusolo, P.; et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European group for blood and marrow transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide: DMSO side effects in autologous transplantation. Transfusion 2014, 54, 2514–2522. [Google Scholar] [CrossRef]
- Mitrus, I.; Smagur, A.; Giebel, S.; Gliwinska, J.; Prokop, M.; Glowala-Kosinska, M.; Chwieduk, A.; Sadus-Wojciechowska, M.; Tukiendorf, A.; Holowiecki, J. A faster reconstitution of hematopoiesis after autologous transplantation of hematopoietic cells cryopreserved in 7.5% dimethyl sulfoxide if compared to 10% dimethyl sulfoxide containing medium. Cryobiology 2013, 67, 327–331. [Google Scholar] [CrossRef]
- Bojanic, I.; Cepulic, B.G.; Mazic, S.; Batinic, D.; Nemet, D.; Labar, B. Toxicity related to autologous peripheral blood haematopoietic progenitor cell infusion is associated with number of granulocytes in graft, gender and diagnosis of multiple myeloma. Vox Sang. 2008, 95, 70–75. [Google Scholar] [CrossRef]
- Calmels, B.; Lemarié, C.; Esterni, B.; Malugani, C.; Charbonnier, A.; Coso, D.; Schiano de Colella, J.-M.; Deconinck, E.; Caillot, D.; Viret, F.; et al. Occurrence and severity of adverse events after autologous hematopoietic progenitor cell infusion are related to the amount of granulocytes in the apheresis product. Transfusion 2007, 47, 1268–1275. [Google Scholar] [CrossRef]
- Cordoba, R.; Arrieta, R.; Kerguelen, A.; Hernandez-Navarro, F. The occurrence of adverse events during the infusion of autologous peripheral blood stem cells is related to the number of granulocytes in the leukapheresis product. Bone Marrow Transplant. 2007, 40, 1063–1067. [Google Scholar] [CrossRef] [Green Version]
- Martín-Henao, G.A.; Resano, P.M.; Villegas, J.M.S.; Manero, P.P.; Sánchez, J.M.; Bosch, M.P.; Codins, A.E.; Bruguera, M.S.; Infante, L.R.; Oyarzabal, A.P.; et al. Adverse reactions during transfusion of thawed haematopoietic progenitor cells from apheresis are closely related to the number of granulocyte cells in the leukapheresis product: Adverse reactions during haematopoietic progenitor cell transfusion. Vox Sang. 2010, 99, 267–273. [Google Scholar] [CrossRef]
- Khera, N.; Jinneman, J.; Storer, B.E.; Heimfeld, S.; O’Meara, M.M.; Chauncey, T.R.; Lee, S.J.; Linenberger, M. Limiting the daily total nucleated cell dose of cryopreserved peripheral blood stem cell products for autologous transplantation improves infusion-related safety with no adverse impact on hematopoietic engraftment. Biol. Blood Marrow Transplant. 2012, 18, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Richa, E. The Correlation between the granulocyte content in infused stem cells and side effects of the infusion. Blood Transfus. 2011, 9, 346. [Google Scholar] [CrossRef]
- Milone, G.; Mercurio, S.; Strano, A.; Leotta, S.; Pinto, V.; Battiato, K.; Coppoletta, S.; Murgano, P.; Farsaci, B.; Privitera, A.; et al. Adverse events after infusions of cryopreserved hematopoietic stem cells depend on non-mononuclear cells in the infused suspension and patient age. Cytotherapy 2007, 9, 348–355. [Google Scholar] [CrossRef]
- Mulay, S.B.; Greiner, C.W.; Mohr, A.; Bryant, S.C.; Lingineni, R.K.; Padley, D.; Hogan, W.J.; Gastineau, D.A.; Jacob, E.K. Infusion technique of hematopoietic progenitor cells and related adverse events (CME): HPC infusion-related AEs. Transfusion 2014, 54, 1997–2003. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Sempek, D.S.; Carey, S.; Grossman, B.J. Adverse events of cryopreserved hematopoietic stem cell infusions in adults: A single-center observational study. Transfusion 2017, 57, 1522–1526. [Google Scholar] [CrossRef] [Green Version]
- FACT-JACIE HEMATOPOIETIC CELLULAR THERAPY Accreditation Manual; FACT-JACIE International Standards; Eighth Edition Version 8.2; Foundation For The Accreditation of Cellular Therapy (FACT): Omaha, NE, USA; Joint Accreditation Committee -ISCT and EBMT (JACIE): Barcelona, Spain, 2021.
- European Parliament. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on Setting Standards of Quality and Safety for the Donation, Procurement, Testing, Processing, Preservation, Storage and Distribution of Human Tissues and Cells; European Parliament: Strasbourg, France, 2009. [Google Scholar]
- Non-Serious Adverse Event Definition. Available online: https://www.lawinsider.com/dictionary/non-serious-adverse-event (accessed on 1 February 2023).
- Vidula, N.; Villa, M.; Helenowski, I.B.; Merchant, M.; Jovanovic, B.D.; Meagher, R.; Mehta, J.; Singhal, S.; Winter, J.N.; Frankfurt, O.; et al. Adverse events during hematopoietic stem cell infusion: Analysis of the Infusion product. Clin. Lymphoma Myeloma Leuk. 2015, 15, e157–e162. [Google Scholar] [CrossRef]
- Vaxman, I.; Visram, A.; Kumar, S.; Dispenzieri, A.; Buadi, F.; Dingli, D.; Lacy, M.; Muchtar, E.; Kapoor, P.; Hogan, W.; et al. Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents. Bone Marrow Transplant. 2021, 56, 1144–1150. [Google Scholar] [CrossRef]
- Er, J.; Routledge, D.; Hempton, J.; Wood, C.; Joyce, T.; Harrison, S.; Campbell, P. Autologous stem cell transplantation in elderly multiple myeloma patients aged ≥ 65 Years: A two-centre australian experience. Intern. Med. J. 2021, 51, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Kawamura, K.; Hanamura, I.; Sunami, K.; Mori, T.; Nakamura, F.; Iida, S.; Nakazawa, H.; Makita, M.; Kako, S.; et al. Efficacy and safety of autologous stem cell transplantation in patients aged ≥ 65 years with multiple myeloma in the era of novel agents. Bone Marrow Transplant. 2019, 54, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Dingli, D.; Kumar, S.; Buadi, F.K.; Dispenzieri, A.; Hayman, S.R.; Wolf, R.C.; Gastineau, D.A.; Chakraborty, R.; Hogan, W.J.; et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone Marrow Transplant. 2016, 51, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Mitrus, I.; Smagur, A.; Fidyk, W.; Czech, M.; Prokop, M.; Chwieduk, A.; Glowala-Kosinska, M.; Czerw, T.; Sobczyk-Kruszelnicka, M.; Mendrek, W.; et al. Reduction of DMSO concentration in cryopreservation mixture from 10% to 7.5% and 5% has no impact on engraftment after autologous peripheral blood stem cell transplantation: Results of a prospective, randomized study. Bone Marrow Transplant. 2018, 53, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Truong, T.H.; Moorjani, R.; Dewey, D.; Guilcher, G.M.T.; Prokopishyn, N.L.; Lewis, V.A. Adverse reactions during stem cell infusion in children treated with autologous and allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 680–686. [Google Scholar] [CrossRef] [Green Version]
- To, L.B.; Levesque, J.-P.; Herbert, K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood 2011, 118, 4530–4540. [Google Scholar] [CrossRef]
- Berger, M.G.; Berger, J.; Richard, C.; Jeanpierre, S.; Nicolini, F.E.; Tournilhac, O.; Michallet, M.; Satta, V.M. Preferential sensitivity of hematopoietic (HPs) and mesenchymal (MPs) progenitors to fludarabine suggests impaired bone marrow niche and HP mobilization. Leukemia 2008, 22, 2131–2134. [Google Scholar] [CrossRef] [Green Version]
- Calado, R.T.; Young, N.S. Telomere maintenance and human bone marrow failure. Blood 2008, 111, 4446–4455. [Google Scholar] [CrossRef]
- Schultz, M.B.; Sinclair, D.A. When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development 2016, 143, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Baerlocher, G.M.; Rice, K.; Vulto, I.; Lansdorp, P.M. Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age: Stem cell turnover and telomere length. Aging Cell 2007, 6, 121–123. [Google Scholar] [CrossRef]
- Van Ziffle, J.A.G.; Baerlocher, G.M.; Lansdorp, P.M. Telomere length in subpopulations of human hematopoietic cells. Stem Cells 2003, 21, 654–660. [Google Scholar] [CrossRef]
- Aubert, G.; Baerlocher, G.M.; Vulto, I.; Poon, S.S.; Lansdorp, P.M. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012, 8, e1002696. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, M.; Asai, O.; Kuraishi, Y.; Urashima, M.; Hoshi, Y.; Sakamaki, H.; Yabe, H.; Furukawa, T.; Yamada, O.; Mizoguchi, H.; et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000, 25, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Baerlocher, G.M.; Rovó, A.; Müller, A.; Matthey, S.; Stern, M.; Halter, J.; Heim, D.; Rischewski, J.; Gratwohl, A.; Tichelli, A. Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: The role of chronic graft-versus-host disease and female donor sex. Blood 2009, 114, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, X.; Zhang, X.; Gao, L.; Kong, P.; Wang, Q.; Peng, X.; Liu, H. Mobilization of peripheral blood stem cells for autologous transplantation patients with hematological malignancies: Influence of disease, mobilization method, age and sex. Transfus. Apher. Sci. 2008, 39, 21–28. [Google Scholar] [CrossRef]
- Mohty, M.; Hübel, K.; Kröger, N.; Aljurf, M.; Apperley, J.; Basak, G.W.; Bazarbachi, A.; Douglas, K.; Gabriel, I.; Garderet, L.; et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: A position statement from the european group for blood and marrow transplantation. Bone Marrow Transplant. 2014, 49, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.F.; Sánchez-Ortega, I. HSCT in elderly patients. In The EBMT Handbook; Carreras, E., Dufour, C., Mohty, M., Kröger, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 499–503. ISBN 978-3-030-02277-8. [Google Scholar]
- Morris, C.L.; Siegel, E.; Barlogie, B.; Cottler-Fox, M.; Lin, P.; Fassas, A.; Zangari, M.; Anaissie, E.; Tricot, G. Mobilization of CD34 + cells in elderly patients (≥70 Years) with multiple myeloma: Influence of age, prior therapy, platelet count and mobilization regimen: Age and CD34 + cell collection in multiple myeloma. Br. J. Haematol. 2003, 120, 413–423. [Google Scholar] [CrossRef]
- Jones, J.A.; Qazilbash, M.H.; Shih, Y.-C.T.; Cantor, S.B.; Cooksley, C.D.; Elting, L.S. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: Clinical and economic outcomes from the nationwide inpatient sample. Cancer 2008, 112, 1096–1105. [Google Scholar] [CrossRef]
- Holbro, A.; Ahmad, I.; Cohen, S.; Roy, J.; Lachance, S.; Chagnon, M.; LeBlanc, R.; Bernard, L.; Busque, L.; Roy, D.C.; et al. Safety and cost-effectiveness of outpatient autologous stem cell transplantation in patients with multiple myeloma. Biol. Blood Marrow Transplant. 2013, 19, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Sauer-Heilborn, A.; Kadidlo, D.; McCullough, J. Patient care during infusion of hematopoietic progenitor cells: Patient care during infusion of HPCs. Transfusion 2004, 44, 907–916. [Google Scholar] [CrossRef]
- Holbro, A.; Baldomero, H.; Lanza, F.; Chabannon, C.; Snowden, J.A.; Buser, A.; Infanti, L.; Worel, N.; Sureda, A.; Badoglio, M.; et al. Handling, processing and disposal of stem cell products in Europe: A survey by the cellular therapy and immunobiology working party of the european society for blood and marrow transplantation. Cytotherapy 2018, 20, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Martinez, C.; Majhail, N.S.; Prestegaard, M.; Maiers, M.; Horwitz, M.; Komanduri, K. Stem cell transplantation and informatics: Current considerations. Biol. Blood Marrow Transplant. 2018, 24, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Patients | n = 449 |
|---|---|
| Gender, n (%) | |
| • Male/Female | 305 (68)/144 (32) |
| Age at 1st transplantation in years | |
| (median/range) | 60/19–79 |
| Diseases, n (%) | |
| • Plasma cell disorder | 208 (46) |
| • Non-Hodgkin lymphoma | 164 (37) |
| • Acute myeloid leukemia | 44 (10) |
| • Other diseases | 33 (7) |
| No. of Patients | No. of Separate Transplantations | Total No. of Transplantations (Thereof CD34+-Selections) | No. of Infusions per Transplantation |
|---|---|---|---|
| 339 | 1 | 339 (80) | 1 |
| 37 | 1 | 37 | 2 |
| 16 | 1 | 16 | 3 |
| 6 | 1 | 6 | 4 |
| 3 | 1 | 3 | 5 |
| 30 | 2 | 60 (2) | 1 |
| 1 | 2 | 2 | 1 and 2 |
| 3 | 2 | 6 | 2 |
| 1 | 2 | 2 | 4 and 5 |
| 13 | 3 | 39 | 1 |
| Total: 449 | Total: 510 |
| Variables | Total Transplantations n = 428 | with AE n = 79 | without AE n = 349 | p |
|---|---|---|---|---|
| Collection and processing | ||||
| • Total volume collected [mL] 1,2 | 287/89–700 | 374/156–700 | 273/89–573 | <0.0001 **** |
| • Lc in transplant product [×109/L] 1,2 | 200/128–431 | 201/146–225 | 200/128–431 | 0.8990 |
| • MNC in transplant product [×109/L] 1,2 | 114/29–231 | 110/53–174 | 115/29–231 | 0.3189 |
| • PNC in transplant product [×109/L] 1,2 | 86/22–199 | 90/30–153 | 86/22–199 | 0.3041 |
| • Total CD34+ cells in transplant product [×106/kg BW] 1 | 8.7/2–29.6 | 6.2/2.1–18.4 | 9.6/2.0–29.6 | <0.0001 **** |
| Transplantation | ||||
| • Age of patients at transplantation [years] 1 | 60/19–79 | 62/24–75 | 58/19–79 | 0.0013 ** |
| • Volume infused [mL] 1 | 220/40–1680 | 310/70–1680 | 180/40–1440 | <0.0001 **** |
| • Volume of DMSO infused [mL] 1 | 11.0/2.0–84.0 | 15.5/3.5–84.0 | 9.0/2.0–72.0 | <0.0001 **** |
| • Neutrophil recovery [days] 1,3,5 | 11/1–32 | 12/8–20 | 11/1–32 | 0.0182 * |
| • Platelet recovery [days] 1,4,6 | 14/10–138 | 15/11–138 | 14/10–108 | 0.0120 * |
| • Body temperature ≥ 38.5 °C [yes/no/n.a. (% of total)] | 401/25/2 (94) | 71/7/1 (89) | 330/18/1 (95) | ‡ 0.20/0.19 |
| • Duration of hospitalization [days] 1 | 21/1–71 | 22/1–71 | 21/1–65 | 0.0436 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haubitz, M.; von Petersdorff, V.S.; Helsen, I.; Brunold, C.; Oppliger Leibundgut, E.; Baerlocher, G.M. Higher Age (≥60 Years) Increases the Risk for Adverse Events during Autologous Hematopoietic Stem Cell Transplantation. Cancers 2023, 15, 1584. https://doi.org/10.3390/cancers15051584
Haubitz M, von Petersdorff VS, Helsen I, Brunold C, Oppliger Leibundgut E, Baerlocher GM. Higher Age (≥60 Years) Increases the Risk for Adverse Events during Autologous Hematopoietic Stem Cell Transplantation. Cancers. 2023; 15(5):1584. https://doi.org/10.3390/cancers15051584
Chicago/Turabian StyleHaubitz, Monika, Vittoria S. von Petersdorff, Ingrid Helsen, Claudio Brunold, Elisabeth Oppliger Leibundgut, and Gabriela M. Baerlocher. 2023. "Higher Age (≥60 Years) Increases the Risk for Adverse Events during Autologous Hematopoietic Stem Cell Transplantation" Cancers 15, no. 5: 1584. https://doi.org/10.3390/cancers15051584
APA StyleHaubitz, M., von Petersdorff, V. S., Helsen, I., Brunold, C., Oppliger Leibundgut, E., & Baerlocher, G. M. (2023). Higher Age (≥60 Years) Increases the Risk for Adverse Events during Autologous Hematopoietic Stem Cell Transplantation. Cancers, 15(5), 1584. https://doi.org/10.3390/cancers15051584