Systematic Review and Meta-Analysis of Oral Anticoagulant Therapy in Atrial Fibrillation Cancer Patients
Abstract
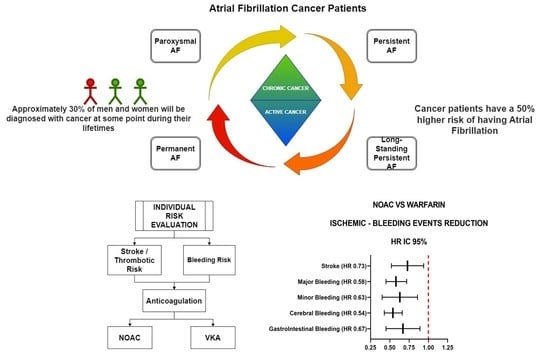
:Simple Summary
Abstract
1. Introduction
2. Purpose of the Study
3. Study Design
4. Search Strategy
5. Study Selection
6. Outcome and Endpoints
7. Bias Assessment
8. Statistical Analysis
9. Results
10. Discussion
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yun, J.P.; Choi, E.K.; Han, K.D.; Park, S.H.; Jung, J.H.; Park, S.H.; Ahn, H.J.; Lim, J.H.; Lee, S.R.; Oh, S. Risk of Atrial Fibrillation According to Cancer Type: A Nationwide Population-Based Study. JACC CardioOncol. 2021, 3, 221–232. [Google Scholar] [CrossRef]
- Falanga, A.; Rickles, F.R. Pathophysiology of the Thrombophilic State in the Cancer Patient. Semin. Thromb. Hemost. 1999, 25, 173–182. [Google Scholar] [CrossRef]
- Johnstone, C.; Rich, S.E. Bleeding in cancer patients and its treatment: A review. Ann. Palliat. Med. 2018, 7, 265–273. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Farmakis, D. Anticoagulation for atrial fibrillation in active cancer: What the cardiologists think. Eur. J. Prev. Cardiol. 2020, 28, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Fanola, C.L.; Ruff, C.T.; Murphy, S.A.; Jin, J.; Duggal, A.; Babilonia, N.A.; Sritara, P.; Mercuri, M.F.; Kamphuisen, P.W.; Antman, E.M.; et al. Efficacy and Safety of Edoxaban in Patients With Active Malignancy and Atrial Fibrillation: Analysis of the ENGAGE AF-TIMI 48 Trial. J. Am. Heart Assoc. 2018, 7, e008987. [Google Scholar] [CrossRef]
- Melloni, C.; Dunning, A.; Granger, C.B.; Thomas, L.; Khouri, M.G.; Garcia, D.A.; Hylek, E.M.; Hanna, M.; Wallentin, L.; Gersh, B.J.; et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and a History of Cancer: Insights from the ARISTOTLE Trial. Am. J. Med. 2017, 130, 1440–1448.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Hellkamp, A.S.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.; Hacke, W.; Halperin, J.L.; Hankey, G.; Mahaffey, K.W.; et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: Observations from ROCKET AF. Eur. Heart J.-Qual. Care Clin. Outcomes 2018, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, Y.-J.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Effect of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Newly Diagnosed Cancer. Korean Circ. J. 2018, 48, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.C.; Kumar, A.; Mccray, W.; Tetewsky, S.; Parone, L.; Sridhara, S.; Prakash, M.P.H.; Tse, G.; Liu, T.; Kanwar, N.; et al. Superior safety of direct oral anticoagulants compared to Warfarin in patients with atrial fibrillation and underlying cancer: A national veterans affairs database study. J. Geriatr. Cardiol. 2019, 16, 706–709. [Google Scholar] [CrossRef]
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018, 2, 200–209. [Google Scholar] [CrossRef]
- Wu, V.C.-C.; Wang, C.-L.; Huang, Y.-T.; Lan, W.-C.; Wu, M.; Kuo, C.-F.; Chen, S.-W.; Chu, P.-H.; Wen, M.-S.; Kuo, C.-C.; et al. Novel Oral Anticoagulant versus Warfarin in Cancer Patients with Atrial Fibrillation: An 8-Year Population-Based Cohort Study. J. Cancer 2020, 11, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Shioyama, W.; Oboshi, M.; Oka, T.; Fujita, M. Oral Anticoagulants in Japanese Patients with Atrial Fibrillation and Active Cancer. Intern. Med. 2019, 58, 1845–1849. [Google Scholar] [CrossRef]
- Potter, A.S.; Patel, A.; Khawaja, M.; Chen, C.; Zheng, H.; Kaczmarek, J.; Gao, F.; Karimzad, K.; Song, J.; Koutroumpakis, E.; et al. Outcomes by Class of Anticoagulant Use for Nonvalvular Atrial Fibrillation in Patients with Active Cancer. JACC: CardioOncology 2022, 4, 341–350. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Keshishian, A.V.; Zhang, Y.; Kang, A.; Dhamane, A.D.; Luo, X.; Klem, C.; Ferri, M.; Jiang, J.; Yuce, H.; et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients with Active Cancer. JACC: CardioOncology 2021, 3, 411–424. [Google Scholar] [CrossRef]
- Ording, A.G.; Søgaard, M.; Skjøth, F.; Grove, E.L.; Lip, G.Y.H.; Larsen, T.B.; Nielsen, P.B. Bleeding complications in patients with gastrointestinal cancer and atrial fibrillation treated with oral anticoagulants. Cancer Med. 2021, 10, 4405–4414. [Google Scholar] [CrossRef] [PubMed]
- Leader, A.; Mendelson Cohen, N.; Afek, S.; Jaschek, R.; Frajman, A.; Itzhaki Ben Zadok, O.; Raanani, P.; Lishner, M.; Spectre, G. Arterial Thromboembolism in Patients with AF and CHA2DS2-VASc Score 0-2 With and Without Cancer. Cardio Oncol. 2023, 5, 174–185. [Google Scholar] [CrossRef]
- Fradley, M.G.; Ellenberg, K.; Alomar, M.; Swanson, J.; Kharod, A.; Nguyen, A.T.H.; Khodor, S.; Mishra, S.; Duong, L.M.; Shah, N.; et al. Patterns of Anticoagulation Use in Patients with Cancer With Atrial Fibrillation and/or Atrial Flutter. JACC CardioOncol. 2020, 2, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Nattel, S.; Macle, L.; Andrade, J.G. Management of Atrial Fibrillation in 2021: An Updated Comparison of the Current CCS/CHRS, ESC, and AHA/ACC/HRS Guidelines. Can. J. Cardiol. 2021, 37, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Shehab, N.; Lovegrove, M.C.; Geller, A.I.; Rose, K.O.; Weidle, N.J.; Budnitz, D.S. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013-2014. JAMA 2016, 316, 2115–2125. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Eur. 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Tummala, R.; Kavtaradze, A.; Gupta, A.; Ghosh, R.K. Specific antidotes against direct oral anticoagulants: A comprehensive review of clinical trials data. Int. J. Cardiol. 2016, 214, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Delluc, A.A.; Wang, T.; Yap, E.; Ay, C.; Schaefer, J.; Carrier, M.; Noble, S. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1247–1252. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Cereda, A.; Walther, C.; Aslam, A. Multidisciplinary Integrated Care in Atrial Fibrillation (MICAF): A Systematic Review and Meta-Analysis. Clin. Med. Res. 2022, 20, 219–230. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EP Eur. 2020, 15, 1133–1180. [Google Scholar]
- Isogai, T.; Saad, A.M.; Abushouk, A.I.; Shekhar, S.; Kuroda, S.; Gad, M.M.; Wazni, O.M.; Krishnaswamy, A.; Kapadia, S.R. Procedural and Short-Term Outcomes of Percutaneous Left Atrial Appendage Closure in Patients with Cancer. Am. J. Cardiol. 2020, 141, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Nielsen, P.B.; Skjøth, F.; Rasmussen, L.H.; Lip, G.Y.H. Non-Vitamin K Antagonist Oral Anticoagulants and the Treatment of Venous Thromboembolism in Cancer Patients: A Semi Systematic Review and Meta-Analysis of Safety and Efficacy Outcomes. PLoS ONE 2014, 9, e114445. [Google Scholar] [CrossRef] [PubMed]
- EINSTEIN Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Faculty Opinions recommendation of Oral rivaroxaban for symptomatic venous thromboembolism. Randomized Control. Trial 2011, 363, 2499–2510. [Google Scholar] [CrossRef]









| Study | Mean Age | Female Sex % | CHA2DS2-VASc | Has-Bled | Apixaban | Dabigatran | Rivaroxaban | Edoxaban | Active Cancer | Follow-Up (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Melloni | 75.00 | 32.00 | 3.80 | 2.2 | 100.00 | - | - | - | 12.70% | 1.80 |
| Shah | 74.70 | 40.00 | 4.40 | - | 17.70 | 36.00 | 46.00 | - | 100% | 4.00 |
| Kim | 70.80 | 45.80 | 3.60 | 1.90 | 35.60 | 36.10 | 36.10 | - | 100% | 1.70 |
| Fanola | 75.00 | 31.10 | 2.80 | 2.7 | - | - | - | 100.00 | 100% | 2.80 |
| Chen | 77.00 | 34.00 | 3.50 | 2.9 | - | - | 100.00 | - | 7.80% | 1.90 |
| Yashui | 72.00 | 14.00 | 3.10 | 2 | 36.20 | 19.70 | 34.60 | 9.40 | 100% | 1.00 |
| Sawant | 76.00 | 20.00 | - | - | 26.1 | 41.2 | 32.7 | - | 100% | 1.00 |
| Chia | 69.90 | 38.00 | 4.10 | 3.30 | - | - | - | - | 100% | 1.50 |
| Deitelzweig | 77.00 | 39.00 | 4.00 | 3.50 | 24.00 | 7.00 | 31.00 | - | 100% | 2.00 |
| Ording | 78.25 | 41.20 | 3.75 | 2.00 | 40.90 | 22.40 | 35.80 | 0.90 | 43% | 1.00 |
| Potter | 71.50 | 71.00 | 3.40 | 1.90 | 57.20 | 6.40 | 36.00 | 0.40 | 100% | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cereda, A.; Lucreziotti, S.; Franchina, A.G.; Laricchia, A.; De Regibus, V.; Conconi, B.; Carlà, M.; Spangaro, A.; Rocchetti, M.; Ponti, L.; et al. Systematic Review and Meta-Analysis of Oral Anticoagulant Therapy in Atrial Fibrillation Cancer Patients. Cancers 2023, 15, 2574. https://doi.org/10.3390/cancers15092574
Cereda A, Lucreziotti S, Franchina AG, Laricchia A, De Regibus V, Conconi B, Carlà M, Spangaro A, Rocchetti M, Ponti L, et al. Systematic Review and Meta-Analysis of Oral Anticoagulant Therapy in Atrial Fibrillation Cancer Patients. Cancers. 2023; 15(9):2574. https://doi.org/10.3390/cancers15092574
Chicago/Turabian StyleCereda, Alberto, Stefano Lucreziotti, Antonio Gabriele Franchina, Alessandra Laricchia, Valentina De Regibus, Barbara Conconi, Matteo Carlà, Andrea Spangaro, Matteo Rocchetti, Luca Ponti, and et al. 2023. "Systematic Review and Meta-Analysis of Oral Anticoagulant Therapy in Atrial Fibrillation Cancer Patients" Cancers 15, no. 9: 2574. https://doi.org/10.3390/cancers15092574
APA StyleCereda, A., Lucreziotti, S., Franchina, A. G., Laricchia, A., De Regibus, V., Conconi, B., Carlà, M., Spangaro, A., Rocchetti, M., Ponti, L., Minardi, A., Sala, E., Sangiorgi, G. M., Tumminello, G., Barbieri, L., Carugo, S., & Aseni, P. (2023). Systematic Review and Meta-Analysis of Oral Anticoagulant Therapy in Atrial Fibrillation Cancer Patients. Cancers, 15(9), 2574. https://doi.org/10.3390/cancers15092574