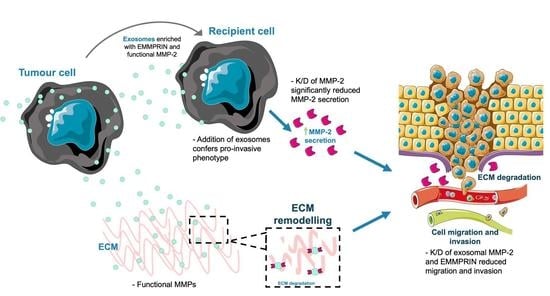
Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. PrestoBlue® Assay
2.3. Isolation of Exosomes
2.4. Migration and Invasion Assays
2.5. Generation of Stable Knockdown Cell Lines
2.6. Preparation of Cerebrospinal Fluid Samples
2.7. Detection of Metalloproteinase Activity by Zymography
2.8. Western Blot
2.9. Quantitative Real-Time Polymerase Chain Reaction
2.10. Transmission Electron Microscopy
2.11. NanoFCM
2.12. Nanoparticle Tracking Analysis
2.13. Statistical Analysis
2.14. Bioinformatic Analysis of Published Datasets
3. Results
3.1. Metastatic Cell Lines Release More Exosomes Than Their Primary Counterparts
3.2. Treatment of Medulloblastoma Cells with Migratory-Derived Exosomes Enhances Cell Invasion and Migration
3.3. MMP-2 and EMMPRIN Are Abundant in Proinvasive Exosomes
3.4. Stimulation of Medulloblastoma Cells with Migratory-Derived Exosomes Enhances Cell Invasion and Migration
3.5. Knockdown of MMP-2 and EMMPRIN Reduces Exosome-Mediated Migration and Invasion
3.6. CSF Levels of Functionally Active MMP2 Increase at Disease Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, R.M.; Richardson, S.; Schwalbe, E.C.; Hicks, D.; Lindsey, J.C.; Crosier, S.; Rafiee, G.; Grabovska, Y.; Wharton, S.B.; Jacques, T.S.; et al. Time, Pattern, and Outcome of Medulloblastoma Relapse and Their Association with Tumour Biology at Diagnosis and Therapy: A Multicentre Cohort Study. Lancet Child Adolesc. Health 2020, 4, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Magaña, S.M.; Peterson, T.E.; Evans, J.E.; Decker, P.A.; Simon, V.; Eckel-Passow, J.E.; Daniels, D.J.; Parney, I.F. Pediatric Brain Tumor Cell Lines Exhibit MiRNA-Depleted, Y RNA-Enriched Extracellular Vesicles. J. Neurooncol. 2022, 156, 269–279. [Google Scholar] [CrossRef]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.T.; Graner, M.W. Medulloblastoma Exosome Proteomics Yield Functional Roles for Extracellular Vesicles. PLoS ONE 2012, 7, e42064. [Google Scholar] [CrossRef] [PubMed]
- Bisaro, B.; Mandili, G.; Poli, A.; Piolatto, A.; Papa, V.; Novelli, F.; Cenacchi, G.; Forni, M.; Zanini, C. Proteomic Analysis of Extracellular Vesicles from Medullospheres Reveals a Role for Iron in the Cancer Progression of Medulloblastoma. Mol. Cell. Ther. 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Huang, S.; Xue, P.; Han, X.; Zhang, C.; Yang, L.; Liu, L.; Wang, X.; Li, H.; Fu, J.; Zhou, Y. Exosomal MiR-130b-3p Targets SIK1 to Inhibit Medulloblastoma Tumorigenesis. Cell Death Dis. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.T.; Wang, S.; Huang, S.L.; Zheng, Y.; Wang, C.Q.; Hu, B.Y.; Qin, W.; Zou, T.T.; Fu, Y.; et al. Isolation and Characterization of Exosomes for Cancer Research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Li, Z.B.; Chen, X.; Yi, X. Tumor Promoting Effects of Exosomal MicroRNA-210 Derived from Lung Cancer Cells on Lung Cancer through the RUNX3/PI3K/AKT Signaling Pathway Axis. J. Biol. Regul. Homeost. Agents 2021, 35, 473–484. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bo, X.; Yi, X.; Xiao, X.; Zheng, Q.; Ma, L.; Li, B. Exosome-Transferred LINC01559 Promotes the Progression of Gastric Cancer via PI3K/AKT Signaling Pathway. Cell Death Dis. 2020, 11, 723. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhang, Y.; Zhang, S.; Qiu, L.; Zhuang, Z.; Wei, M.; Deng, X.; Wang, Z.; Han, J. The Key Role of Exosomes on the Pre-Metastatic Niche Formation in Tumors. Front. Mol. Biosci. 2021, 8, 703640. [Google Scholar] [CrossRef] [PubMed]
- Kogure, A.; Yoshioka, Y.; Ochiya, T. Extracellular Vesicles in Cancer Metastasis: Potential as Therapeutic Targets and Materials. Int. J. Mol. Sci. 2020, 21, 4463. [Google Scholar] [CrossRef] [PubMed]
- Aili, Y.; Maimaitiming, N.; Qin, H.; Ji, W.; Fan, G.; Wang, Z.; Wang, Y. Tumor Microenvironment and Exosomes in Brain Metastasis: Molecular Mechanisms and Clinical Application. Front. Oncol. 2022, 12, 5684. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, A.; Miękus, N.; Stefanowicz, J.; Adamkiewicz-Drożyńska, E. Selected Matrix Metalloproteinases (MMP-2, MMP-7) and Their Inhibitor (TIMP-2) in Adult and Pediatric Cancer. Diagnostics 2020, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zeng, X.; Gu, H.; Li, M.; Tan, H.; Jin, Z.; Hua, T.; Shi, R.; Wang, H. CD147/EMMPRIN Overexpression and Prognosis in Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 32804. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Al-Rubai, A.J.; Grabowska, A.M.; Pratten, M.K. Separating Chemotherapy-Related Developmental Neurotoxicity from Cytotoxicity in Monolayer and Neurosphere Cultures of Human Fetal Brain Cells. Toxicol. In Vitro 2016, 37, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and Efficiency Assessment of Six Extracellular Vesicle Isolation Methods by Nano-Flow Cytometry. J. Extracell Vesicles 2020, 9, 1697028. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-Generation Molecular Subgrouping of Medulloblastoma: An International Meta-Analysis of Group 3 and Group 4 Subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular Subgroups of Medulloblastoma: The Current Consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Northcott, P.A.; Jones, D.T.W.; Kool, M.; Robinson, G.W.; Gilbertson, R.J.; Cho, Y.J.; Pomeroy, S.L.; Korshunov, A.; Lichter, P.; Taylor, M.D.; et al. Medulloblastomics: The End of the Beginning. Nat. Rev. Cancer 2012, 12, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Prim. 2019, 5, 11. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Théry, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and Characterization of Exosomes from Cell Culture Supernatants. Curr. Protoc. Cell Biol. 2006, 30, 2–22. [Google Scholar] [CrossRef] [PubMed]
- van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Baran, J.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Szatanek, R.; Zembela, M.; Barbasz, J.; Czupryna, A.; Szczepanik, A.; Zembala, M. Circulating Tumour-Derived Microvesicles in Plasma of Gastric Cancer Patients. Cancer Immunol. Immunother. 2010, 59, 841–850. [Google Scholar] [CrossRef]
- Yamamoto, H.; Watanabe, Y.; Oikawa, R.; Morita, R.; Yoshida, Y.; Maehata, T.; Yasuda, H.; Itoh, F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA Is a Useful Marker for Early Detection of Gastric Cancer in an H. Pylori -Independent Manner. Clin. Transl. Gastroenterol. 2016, 7, e184. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sheng, B.; Zeng, Q.; Yao, W.; Jiang, Q. Correlation between MMP2 Expression in Lung Cancer Tissues and Clinical Parameters: A Retrospective Clinical Analysis. BMC Pulm. Med. 2020, 20, 283. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52. [Google Scholar] [CrossRef] [PubMed]
- Sier, C.F.M.; Zuidwijk, K.; Zijlmans, H.J.M.A.A.; Hanemaaijer, R.; Mulder-Stapel, A.A.; Prins, F.A.; Dreef, E.J.; Kenter, G.G.; Fleuren, G.J.; Gorter, A. EMMPRIN-Induced MMP-2 Activation Cascade in Human Cervical Squamous Cell Carcinoma. Int. J. Cancer 2006, 118, 2991–2998. [Google Scholar] [CrossRef]
- Menck, K.; Scharf, C.; Bleckmann, A.; Dyck, L.; Rost, U.; Wenzel, D.; Dhople, V.M.; Siam, L.; Pukrop, T.; Binder, C.; et al. Tumor-Derived Microvesicles Mediate Human Breast Cancer Invasion through Differentially Glycosylated EMMPRIN. J. Mol. Cell Biol. 2015, 7, 143–153. [Google Scholar] [CrossRef]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 Production through CD147/Extracellular Matrix Metalloproteinase Inducer Interactions. Cancer Res. 2001, 61, 2276–2281. [Google Scholar] [PubMed]
- Fahs, A.; Hussein, N.; Zalzali, H.; Ramadan, F.; Ghamloush, F.; Tamim, H.; el Homsi, M.; Badran, B.; Boulos, F.; Tawil, A.; et al. CD147 Promotes Tumorigenesis via Exosome-Mediated Signaling in Rhabdomyosarcoma. Cells 2022, 11, 2267. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, N.W.; Azzam, E.I. Extracellular Vesicles Originating from Glioblastoma Cells Increase Metalloproteinase Release by Astrocytes: The Role of CD147 (EMMPRIN) and Ionizing Radiation. Cell Commun. Signal. 2020, 18, 21. [Google Scholar] [CrossRef]
- Chu, T.; Chen, X.; Yu, J.; Xiao, J.; Fu, Z. Extracellular Matrix Metalloproteinase Inducer Is a Negative Prognostic Factor of Pediatric Medulloblastoma. Pathol. Oncol. Res. 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Isaacson, K.J.; Martin Jensen, M.; Subrahmanyam, N.B.; Ghandehari, H. Matrix-Metalloproteinases as Targets for Controlled Delivery in Cancer: An Analysis of Upregulation and Expression. J. Control. Release 2017, 259, 62–75. [Google Scholar] [CrossRef]
- Shimoda, M.; Khokha, R. Metalloproteinases in Extracellular Vesicles. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Redzic, J.S.; Kendrick, A.A.; Bahmed, K.; Dahl, K.D.; Pearson, C.G.; Robinson, W.A.; Robinson, S.E.; Graner, M.W.; Eisenmesser, E.Z. Extracellular Vesicles Secreted from Cancer Cell Lines Stimulate Secretion of MMP-9, IL-6, TGF-Β1 and EMMPRIN. PLoS ONE 2013, 8, e71225. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Higashi, Y.; Fukushige, T.; Baba, N.; Kawai, K.; Hashiguchi, T.; Su, J.; Zeng, W.; Chen, X.; Kanekura, T. Cleaved CD147 Shed from the Surface of Malignant Melanoma Cells Activates MMP2 Produced by Fibroblasts. Anticancer Res. 2014, 34, 7091–7096. [Google Scholar]
- van Ommeren, R.; Garzia, L.; Holgado, B.L.; Ramaswamy, V.; Taylor, M.D. The Molecular Biology of Medulloblastoma Metastasis. Brain Pathol. 2020, 30, 691–702. [Google Scholar] [CrossRef]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. In Advances in Clinical Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 74. [Google Scholar] [CrossRef]
- Angelucci, A.; D’Ascenzo, S.; Festuccia, C.; Gravina, G.L.; Bologna, M.; Dolo, V.; Pavan, A. Vesicle-Associated Urokinase Plasminogen Activator Promotes Invasion in Prostate Cancer Cell Lines. Clin. Exp. Metastasis 2000, 18, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular Transfer of the Oncogenic Receptor EGFRvIII by Microvesicles Derived from Tumour Cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Özen, Ö.; Krebs, B.; Hemmerlein, B.; Pekrun, A.; Kretzschmar, H.; Herms, J. Expression of Matrix Metalloproteinases and Their Inhibitors in Medulloblastomas and Their Prognostic Relevance. Clin. Cancer Res. 2004, 10, 4746–4753. [Google Scholar] [CrossRef]
- Mateo, E.C.; Motta, F.J.N.; Queiroz, R.G.P.; Scrileli, C.A.; Tone, L.G. Protein Expression of the Matrix Metalloproteinase (MMP-1, -2, -3, -9 and -14) in Ewing Family Tumors and Medulloblastoma of Pediatric Patients. Pediatr. Ther. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Tóth, E.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a Protein Corona on the Surface of Extracellular Vesicles in Blood Plasma. J. Extracell Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef]
- Palviainen, M.I.; Saraswat, M.; Varga, Z.; na Kitka, D.; Neuvonen, M.; Puhka, M.; Joenvä, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular Vesicles from Human Plasma and Serum Are Carriers of Extravesicular Cargo-Implications for Biomarker Discovery. PloS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef] [PubMed]
- Albacete-Albacete, L. Extracellular Vesicles: An Emerging Mechanism Governing the Secretion and Biological Roles of Tenascin-C. Front. Immunol. 2021, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “Matrix Metalloproteinases in Lung Health and Disease” Edited by J. Müller-Quernheim and O. Eickelberg Number 1 in This Series: Biological Role of Matrix Metalloproteinases: A Critical Balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, H.K.; Mitoko, C.; Linke, F.; Macarthur, D.; Kerr, I.D.; Coyle, B. Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner. Cancers 2023, 15, 2601. https://doi.org/10.3390/cancers15092601
Jackson HK, Mitoko C, Linke F, Macarthur D, Kerr ID, Coyle B. Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner. Cancers. 2023; 15(9):2601. https://doi.org/10.3390/cancers15092601
Chicago/Turabian StyleJackson, Hannah K., Christine Mitoko, Franziska Linke, Donald Macarthur, Ian D. Kerr, and Beth Coyle. 2023. "Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner" Cancers 15, no. 9: 2601. https://doi.org/10.3390/cancers15092601
APA StyleJackson, H. K., Mitoko, C., Linke, F., Macarthur, D., Kerr, I. D., & Coyle, B. (2023). Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner. Cancers, 15(9), 2601. https://doi.org/10.3390/cancers15092601