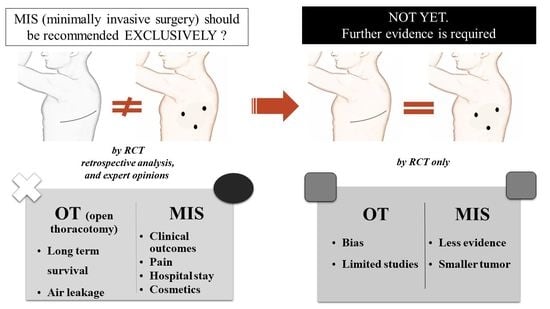
The Uncomfortable Truth: Open Thoracotomy versus Minimally Invasive Surgery in Lung Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Review of the Literature
2.2. Statistical Analysis
2.3. GRADE Approach
3. Results
3.1. Collective Review of the Guidelines
3.2. Randomized Controlled Trials (RCTs) Comparing Open vs. VATS
3.2.1. Patients
- Review of the patient allocation and surgery
- Analysis of the patients’ characteristics (Table 2)
3.2.2. Outcomes
| Study | Operative and Clinical Outcome | Postoperative Complication | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Mortality ⸹ | Operating Time (min) | Hospital Stay (Days) | Hemorrhage | PAL ¶ | Pneumonia | Arrhythmia | Respiratory Failure ⁂ | Chylothorax | ||||||||||
| MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open | |
| Kirby et al., (1995) [13] | mean 161 SD 61 | mean 175 SD 93 | mean 7.1 SD 5.5 | mean 8.3 SD 5.7 | 3/25 | 8/30 | ||||||||||||
| Sugi et al., (2000) [9] | ||||||||||||||||||
| Craig et al., (2001) [14] | mean 141 min (SD 39.5) | mean 121 min (SD 31.4) | mean 8.6 (SD 3.02) | mean 7.9 (SD 3.23) | 1 of 22 | 0/22 | 1/19 | 1/22 | 2/19 | |||||||||
| Palade et al., (2013) [10] | 1/32 | 0/32 | mean 187 SD 38 | mean 158 SD 39 | median 9 range 6–25 | median 11 range 8–36 | 0/32 | 0/32 | 1/32 | 1/32 | 8/32 | 2/32 | 1/32 | 1/32 | 1/32 | 4/32 | 0/32 | 2/32 |
| Bendixen et al., (2016) [8] | 1/102 | 1/99 | median 100 (IQR 80–115) | median 79 min (IQR 60–101) | median 4 days (IQR 2–13) | median 5 days (IQR 2–18) | 14/102 ⸙ | 9/99 ⸙ | 5/102 | 6/99 | 1/102 | 1/99 | ||||||
| Long et al., (2018) [11] | 0/215 | 0/210 | median 150 (IQR 115–195) | median 166 (IQR 130–205) | median 14 (IQR 12–19) | median 15 (IQR 13–19) | 2/215 | 0/210 | 3/215 | 5/210 | 3/215 | 7/210 | 1/215 | 1/210 | 0/215 | 2/210 | ||
| Lim et al., (2022) [7] | 2/221 † | 5/232 † | median 150 (IQR 120–186) | median 132 (IQR 108–168) | median 4 (IQR 3–7) | median 5 (IQR 3–8) | 2/247 | 2/255 | 20/135 | 11/146 | 37/247 | 53/255 | 23/247 | 22/255 | 12/247 | 12/255 | 0/247 | 3/255 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Onaitis, M.W.; D’Amico, T.A.; Chen, H. Minimally Invasive Thoracic Surgery 3.0: Lessons Learned from the History of Lung Cancer Surgery. Ann. Surg. 2018, 267, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus Lobectomy in Small-Sized Peripheral Non-Small-Cell Lung Cancer (Jcog0802/Wjog4607l): A Multicentre, Open-Label, Phase 3, Randomised, Controlled, Non-Inferiority Trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Kim, D.; Kang, G.W.; Jang, H.; Cho, J.Y.; Yang, B.; Yang, H.C.; Hwang, J. Trend of Lung Cancer Surgery, Hospital Selection, and Survival between 2005 and 2016 in South Korea. Thorac. Cancer 2022, 13, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-Assisted Thoracic Surgery versus Video-Assisted Thoracic Surgery for Lung Lobectomy or Segmentectomy in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Sihoe, A.D.L. Uniportal Lung Cancer Surgery: State of the Evidence. Ann. Thorac. Surg. 2019, 107, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dai, Z.; Wei, X.; Pompili, C.; Shi, Q.L.; Xie, T.P.; He, J.T.; Li, Q. Early Patient-Reported Outcomes after Uniportal Vs. Multiportal Thoracoscopic Lobectomy. Ann. Thorac. Surg. 2022, 114, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Batchelor, T.J.P.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1, EVIDoa2100016. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative Pain and Quality of Life after Lobectomy Via Video-Assisted Thoracoscopic Surgery or Anterolateral Thoracotomy for Early Stage Lung Cancer: A Randomised Controlled Trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Sugi, K.; Kaneda, Y.; Esato, K. Video-Assisted Thoracoscopic Lobectomy Achieves a Satisfactory Long-Term Prognosis in Patients with Clinical Stage IA Lung Cancer. World J. Surg. 2000, 24, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Palade, E.; Passlick, B.; Osei-Agyemang, T.; Gunter, J.; Wiesemann, S. Video-Assisted Vs Open Mediastinal Lymphadenectomy for Stage I Non-Small-Cell Lung Cancer: Results of a Prospective Randomized Trial. Eur. J. Cardiothorac. Surg. 2013, 44, 244–249; discussion 49. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Tan, Q.; Luo, Q.; Wang, Z.; Jiang, G.; Situ, D.; Lin, Y.; Su, X.; Liu, Q.; Rong, T. Thoracoscopic Surgery versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. Ann. Thorac. Surg. 2018, 105, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Batchelor, T.; Shackcloth, M.; Dunning, J.; McGonigle, N.; Brush, T.; Dabner, L.; Harris, R.; McKeon, H.E.; Paramasivan, S.; et al. Study Protocol for Video Assisted Thoracoscopic Lobectomy versus Conventional Open Lobectomy for Lung Cancer, a UK Multicentre Randomised Controlled Trial with an Internal Pilot (the Violet Study). BMJ Open 2019, 9, e029507. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.J.; Mack, M.J.; Landreneau, R.J.; Rice, T.W. Lobectomy—Video-Assisted Thoracic Surgery versus Muscle-Sparing Thoracotomy. A Randomized Trial. J. Thorac. Cardiovasc. Surg. 1995, 109, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.R.; Leaver, H.A.; Yap, P.L.; Pugh, G.C.; Walker, W.S. Acute Phase Responses Following Minimal Access and Conventional Thoracic Surgery. Eur. J. Cardiothorac. Surg. 2001, 20, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Valo, J.K.; Kytö, V.; Sipilä, J.; Rautava, P.; Sihvo, E.; Gunn, J. Thoracoscopic Surgery for Lung Cancer Is Associated with Improved Survival and Shortened Admission Length: A Nationwide Propensity-Matched Study. Eur. J. Cardiothorac. Surg. 2020, 57, 100–106. [Google Scholar] [CrossRef]
- Piwkowski, C.; Gabryel, P.; Campisi, A.; Orłowski, T.M.; Zieliński, M.; Rzyman, W.; Kowalewski, J.; Czyżewski, D.; Grochowski, Z.; Wójcik, J.; et al. Ninety-Day Mortality of Thoracoscopic Vs Open Lobectomy: A Large Multicenter Cohort Study. Ann. Thorac. Surg. 2022, 115, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Ichinose, J.; Matsuura, Y.; Nakagawa, K.; Okumura, S.; Mun, M. Long-Term Oncological Outcome after Thoracoscopic Lobectomy for Non-Small Cell Lung Cancer Patients. J. Thorac. Dis. 2019, 11, 3112–3121. [Google Scholar] [CrossRef]
- Bhagat, R.; Bronsert, M.R.; Henderson, W.G.; Scott, C.D.; Weyant, M.J.; Mitchell, J.D.; Fullerton, D.A.; Meguid, R.A. Analysis of Discharge Destination after Open versus Minimally Invasive Surgery for Lung Cancer. Ann. Thorac. Surg. 2020, 109, 375–382. [Google Scholar] [CrossRef]
- Al-Ameri, M.; Bergman, P.; Franco-Cereceda, A.; Sartipy, U. Video-Assisted Thoracoscopic versus Open Thoracotomy Lobectomy: A Swedish Nationwide Cohort Study. J. Thorac. Dis. 2018, 10, 3499–3506. [Google Scholar] [CrossRef]
- Zhang, O.; Alzul, R.; Carelli, M.; Melfi, F.; Tian, D.; Cao, C. Complications of Robotic Video-Assisted Thoracoscopic Surgery Compared to Open Thoracotomy for Resectable Non-Small Cell Lung Cancer. J. Pers Med. 2022, 12, 1311. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Xiao, D.; Zhou, Y.; Chen, Y.; Yang, H.; Wang, L.; Zeng, J.; He, B.; He, R.; et al. Robotic-Assisted Thoracic Surgery Following Neoadjuvant Chemoimmunotherapy in Patients with Stage III Non-Small Cell Lung Cancer: A Real-World Prospective Cohort Study. Front. Oncol. 2022, 12, 969545. [Google Scholar] [CrossRef]
- Casiraghi, M.; Sedda, G.; Diotti, C.; Mariolo, A.V.; Galetta, D.; Tessitore, A.; Maisonneuve, P.; Spaggiari, L. Postoperative Outcomes of Robotic-Assisted Lobectomy in Obese Patients with Non-Small-Cell Lung Cancer. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, R.; Ma, Z.; Han, J.; Yue, W.; Aigner, C.; Casiraghi, M.; Tian, H. Comparison of the Perioperative Outcomes between Robotic-Assisted Thoracic Surgery and Video-Assisted Thoracic Surgery in Non-Small Cell Lung Cancer Patients with Different Body Mass Index Ranges. Transl. Lung Cancer Res. 2022, 11, 1108–1118. [Google Scholar] [CrossRef]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic versus Video-Assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-Analysis. Ann. Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef]
- Huang, J.; Tian, Y.; Zhou, Q.J.; Ning, J.W.; Gu, Z.N.; Lu, P.J.; Li, J.T.; Lin, H.; Chen, T.X.; Yang, Y.H.; et al. Comparison of Perioperative Outcomes of Robotic-Assisted versus Video-Assisted Thoracoscopic Right Upper Lobectomy in Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 4549–4557. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kang, P.; Tao, S.; Li, Q.; Wang, R.; Tan, Q. Cost-Effectiveness Evaluation of Robotic-Assisted Thoracoscopic Surgery versus Open Thoracotomy and Video-Assisted Thoracoscopic Surgery for Operable Non-Small Cell Lung Cancer. Lung Cancer 2021, 153, 99–107. [Google Scholar] [CrossRef]
- Alvarado, C.E.; Worrell, S.G.; Sarode, A.L.; Jiang, B.; Halloran, S.J.; Argote-Greene, L.M.; Linden, P.A.; Towe, C.W. Comparing Thoracoscopic and Robotic Lobectomy Using a Nationally Representative Database. Am. Surg. 2022. online ahead of print. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer, Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 8 March 2023).
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; Esmo Guidelines Committee. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Lung Cancer: Diagnosis and Treatment. Available online: https://www.nice.org.uk/guidance/NG122 (accessed on 8 March 2023).
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.Z.; Deng, H.Y.; Huang, W.; Zhou, Q. Intraoperative Conversion from Video-Assisted Thoracoscopic Lobectomy to Thoracotomy for Non-Small-Cell Lung Cancer: Does It Have an Impact on Long-Term Survival? Interact Cardiovasc. Thorac. Surg. 2022, 35, ivac176. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Hong, J.M.; Kim, D. What Is Difficult About Doing Video-Assisted Thoracic Surgery (VATS)? A Retrospective Study Comparing VATS Anatomical Resection and Conversion to Thoracotomy for Lung Cancer in a University-Based Hospital. J. Thorac. Dis. 2017, 9, 3825–3831. [Google Scholar] [CrossRef]
- Kent, M.S.; Hartwig, M.G.; Vallieres, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (Portal) Study: Survival Analysis of 6646 Cases. Ann. Surg. 2023. online ahead of print. [Google Scholar]
- Kim, D. Invited Editorial on “Intraoperative Conversion During Video-Assisted Thoracoscopy Does Not Constitute a Treatment Failure”. J. Thorac. Dis. 2019, 11, S1231–S1233. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.M.; Berry, M.F.; D’Amico, T.A. Contraindications of Video-Assisted Thoracoscopic Surgical Lobectomy and Determinants of Conversion to Open. J. Thorac. Dis. 2013, 35 (Suppl. S35), S182–S189. [Google Scholar]
- Orri, M.; Revah-Levy, A.; Farges, O. Surgeons’ Emotional Experience of Their Everyday Practice—A Qualitative Study. PLoS ONE 2015, 10, e0143763. [Google Scholar] [CrossRef]
- Yoong, J.; Park, E.R.; Greer, J.A.; Jackson, V.A.; Gallagher, E.R.; Pirl, W.F.; Back, A.L.; Temel, J.S. Early Palliative Care in Advanced Lung Cancer: A Qualitative Study. JAMA Intern. Med. 2013, 173, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Forte, S.; Caronia, F.P.; Ferrigno, F.; Santini, M.; Petersen, R.H.; Fang, W. Is Video-Assisted Thoracoscopic Lobectomy Associated with Higher Overall Costs Compared with Open Surgery? Results of Best Evidence Topic Analysis. Thorac. Cancer 2021, 12, 567–579. [Google Scholar] [CrossRef]
- Chen, W.; Yu, Z.; Zhang, Y.; Liu, H. Comparison of Cost Effectiveness between Video-Assisted Thoracoscopic Surgery (VATS) and Open Lobectomy: A Retrospective Study. Cost Eff. Resour. Alloc. 2021, 19, 55. [Google Scholar] [CrossRef]
- Charvin, M.; Späth, H.M.; Bernard, A.; Bertaux, A.C. A Micro-Costing Evaluation of Lobectomy by Thoracotomy versus Thoracoscopy. J. Thorac. Dis. 2019, 11, 1233–1242. [Google Scholar] [CrossRef]
- Kiladze, I.; Mariamidze, E.; Jeremic, B. Real-World Treatment Patterns of Lung Cancer in a Resource-Restricted Country: The Experience of Georgia. Health Serv. Insights 2021, 14, 11786329211055296. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, M.; Kronborg, C.; Jorgensen, O.D.; Andersen, C.; Licht, P.B. Cost-Utility Analysis of Minimally Invasive Surgery for Lung Cancer: A Randomized Controlled Trial. Eur. J. Cardiothorac. Surg. 2019, 56, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.A.; Smeltzer, M.P.; Faris, N.R.; Osarogiagbon, R.U. Survival after Mediastinal Node Dissection, Systematic Sampling, or Neither for Early Stage NSCLC. J. Thorac. Oncol. 2020, 15, 1670–1681. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U. The Pathologic Nodal Staging Quality Gap: Challenge as Opportunity in Disguise. J. Thorac. Oncol. 2022, 17, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, L.; Wouters, M.; Hartemink, K.J.; Heineman, D.J.; Verhagen, A. Completeness of Lymph Node Dissection in Patients Undergoing Minimally Invasive- or Open Surgery for Non-Small Cell Lung Cancer: A Nationwide Study. Eur. J. Surg. Oncol. 2021, 47, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Heiden, B.T.; Eaton, D.B., Jr.; Chang, S.H.; Yan, Y.; Schoen, M.W.; Patel, M.R.; Kreisel, D.; Nava, R.G.; Meyers, B.F.; Kozower, B.D.; et al. Assessment of Updated Commission on Cancer Guidelines for Intraoperative Lymph Node Sampling in Early Stage NSCLC. J. Thorac. Oncol. 2022, 17, 1287–1296. [Google Scholar] [CrossRef] [PubMed]

| Name | Indication of Surgery | Surgical Extent | Mediastinal Lymph Node | Opinions for MIS |
|---|---|---|---|---|
| Treatment of stage I and II NSCLC; Diagnosis and management of lung cancer, 3rd edition: ACCP guidelines [2] | -Stage I and II | -For clinical stage I and II, a lobectomy is recommended -For stage I predominantly GGO less than 2 cm sublobar resection with negative margin is suggested | -SLND (stage II) or SLNS (stage I) is required | -For stage I, a MIS (VATS) is preferred over a thoracotomy for anatomic pulmonary resection and is suggested in experienced centers (Grade 2C; weak recommendation with low level of evidence). |
| ESMO Clinical Practice guidelines (2017) [3] | -Stage I and II -Single N2 (with neoadjuvant or adjuvant) -Superior sulcus or resectable T3/T4 (with neoadjuvant) | -Lobectomy is still considered the standard operation of tumors over 2 cm -Segmentectomy for pure GGO, AIS, or MIA | -SLND is recommended in Stage II and IIIA | -Either open thoracotomy or VATS access can be carried out as appropriate to the expertise of the surgeon. -Standard open thoracotomy or VATS is probably less important from oncologic perspective. -VATS should be the approach of choice in stage I tumors (V, C; studies without control group, case reports, expert opinions with insufficient evidence for efficacy. |
| NICE Lung cancer: diagnosis and management (March 2019) [4] | -For whom are well enough and for whom treatment with curative intent is suitable | -Offer lobectomy (either open or thoracoscopic) | -SLNS or SLND | -Either open or thoracoscopic |
| NCCN Guidelines: NSCLC (version 2, 2023) [5] | -Stage I and II -Stage IIIA (N2): for resectable cases -T3 (invasion) and T4 local extension tumors | -Anatomic pulmonary resection is preferred. -Segmentectomy (preferred) or wedge is appropriate in selected | -SLNS | -VATS or MIS (including RATS) should be strongly considered for patients with no anatomic or surgical contraindications. -In high-volume centers with significant VATS experience, VATS lobectomy in selected patients results in improved early outcomes (decreased pain, reduced length of hospital stays, more rapid return to function, fewer complications) without compromise of cancer outcomes. -RATS seems to be more expensive than conventional VATS. |
| Author (Year) | Country | Period | Inclusion Criteria | Number of Patients | Excluded for Analysis | Male | Age | Adenocarcinoma | Tumor Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Enrolled | MIS Analyzed | Open Analyzed | MIS | Open | MIS | Open | MIS | Open | MIS | Open | |||||
| Kirby et al., (1995) [13] | USA | 1991–1993 | Clinical stage Ⅰ | 61 | 25 | 30 | Non-malignant lesion, | 13/25 | 13/30 | ||||||
| Sugi et al., (2000) [9] | Japan | 1993–1994 | Clinical stage IA | 100 | 48 | 52 | 28/48 | 29/52 | mean 65.9 SEM 1.4 | mean 64.9 SEM 1.4 | non-squamous 33/48 | non-squamous 41/52 | mean 20.2 mm SEM 1.8 | mean 22.6 mm SEM 1.2 | |
| Craig et al., (2001) [14] | UK | Peripheral opacity lesions | 44 | 22 | 19 | Patients’ refusal | 8/22 | 14/19 | median 64.5 (range 46–78) | median 62 (range 47–74) | 178/215 | 161/210 | |||
| Palade et al., (2013) [10] | Germany | 2008–2011 | Clinical stage Ⅰ | 66 | 32 | 32 | 21/32 | 23/32 | mean 67.7 SD 8.6 | mean 64.7 SD 7.3 | 20/32 | 21/32 | mean 23.5 mm SD 14.4 | mean 24.3 mm SD 13.8 | |
| Bendixen et al., (2016) [8] | Denmark | 2008–2014 | Clinical stage I | 206 | 102 | 99 | Non-malignant lesion, other malignancy | 50/102 | 47/99 | median 66 IQR 62–72 | median 65 IQR 60–72 | 61/102 | 61/99 | ||
| Long et al., (2018) [11] | China | 2008–2014 | Clinical Stage Ⅰ–Ⅱ | 481 | 215 | 210 | SCLC Non-malignant lesion, age > 75 | 105/215 | 107/210 | mean 57.11 SD 9.069 | mean 58.1 SD 9.22 | 178/215 | 161/210 | median 25 mm IQR 17–32 | median 30 mm IQR 20–40 |
| Lim et al., (2022) [7] | UK | 2015–2019 | Clinical stage I–III | 503 | 247 | 255 | 119/247 | 130/255 | Mean 69 SD 8.7 | Mean 69 SD 9.0 | 80/247 | 91/255 | |||
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | VATS | Thoracotomy | Relative (95% CI) | Absolute (95% CI) | ||
| Early mortality | ||||||||||||
| 4 | randomised trials | not serious | not serious | not serious | very serious a | none | 4/570 (0.7%) | 6/573 (1.0%) | RR 0.71 (0.21 to 2.35) | 3 fewer per 1000 (From 8 fewer to 14 more) | ⨁⨁◯◯ Low | CRITICAL |
| Hemorrhage | ||||||||||||
| 5 | randomised trials | serious | not serious | not serious | serious a | Different indication for intervention | 19/618 (3.1%) | 11/615 (1.8%) | RR 1.62 (0.82 to 3.22) | 11 more per 1000 (from 3 fewer to 40 more) | ⨁⨁◯◯ Low | CRITICAL |
| Prolonged air-leakage | ||||||||||||
| 4 | randomised trials | not serious | not serious | not serious | serious a | Different treatments for air-leakage | 29/294 (9.9%) | 26/307 (8.5%) | RR 1.20 (0.73 to 1.99) | 17 more per 1000 (from 23 fewer to 84 more) | ⨁⨁⨁◯ Moderate | IMPORTANT |
| Respiratory Failure | ||||||||||||
| 4 | randomised trials | serious | not serious | not serious | serious a | none | 15/516 (2.9%) | 19/516 (3.7%) | RR 0.80 (0.41 to 1.54) | 7 fewer per 1000 (from 22 fewer to 20 more) | ⨁⨁◯◯ Low | IMPORTANT |
| Arrhythmia | ||||||||||||
| 5 | randomised trials | not serious | not serious | not serious | serious a | none | 28/618 (4.5%) | 32/615 (5.2%) | RR 0.89 (0.55 to 1.44) | 6 fewer per 1000 (from 23 fewer to 23 more) | ⨁⨁⨁◯ Moderate | IMPORTANT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Woo, W.; Shin, J.I.; Lee, S. The Uncomfortable Truth: Open Thoracotomy versus Minimally Invasive Surgery in Lung Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2630. https://doi.org/10.3390/cancers15092630
Kim D, Woo W, Shin JI, Lee S. The Uncomfortable Truth: Open Thoracotomy versus Minimally Invasive Surgery in Lung Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(9):2630. https://doi.org/10.3390/cancers15092630
Chicago/Turabian StyleKim, Dohun, Wongi Woo, Jae Il Shin, and Sungsoo Lee. 2023. "The Uncomfortable Truth: Open Thoracotomy versus Minimally Invasive Surgery in Lung Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 9: 2630. https://doi.org/10.3390/cancers15092630
APA StyleKim, D., Woo, W., Shin, J. I., & Lee, S. (2023). The Uncomfortable Truth: Open Thoracotomy versus Minimally Invasive Surgery in Lung Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(9), 2630. https://doi.org/10.3390/cancers15092630