Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies
Abstract
:Simple Summary
Abstract
1. Introduction
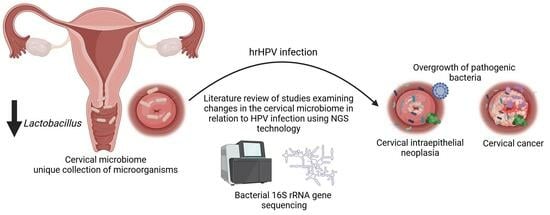
2. HPV Infection and Cervical Cancer
3. Relationship between Vaginal and Cervico-Vaginal Microbiota and HPV Infection
4. Microbial Influence on Cervical Cancer Development: Immune Responses and Therapeutic Prospects
5. Link between Cervical Metabolites and HPV Infection
6. Next-Generation-Sequencing-Based Studies and the Cervical and Cervico-Vaginal Microbiota
7. Evidence from NGS-Based Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.W.B.; Ma, V.Y.; Alcántar-Fernández, J.; Wee, H.L. Is It Time to Genotype Beyond HPV16 and HPV18 for Cervical Cancer Screening? Int. J. Public. Health 2022, 67, 1604621. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Pedretti, F.; Mauramati, S.; Filauro, M.; Vallin, A.; Mora, F.; Crosetti, E.; Succo, G.; Peretti, G.; Benazzo, M. Recurrent Laryngeal Papillomatosis: Multimodal Therapeutic Strategies. Literature Review and Multicentre Retrospective Study. Acta Otorhinolaryngol. Ital. 2023, 43, S111–S122. [Google Scholar] [CrossRef]
- Leslie, S.W.; Sajjad, H.; Kumar, S. Genital Warts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Okunade, K.S. Human Papillomavirus and Cervical Cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; De Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Cervical Cancer Statistics I World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/cervical-cancer-statistics/ (accessed on 23 October 2023).
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Moody, C. Mechanisms by Which HPV Induces a Replication Competent Environment in Differentiating Keratinocytes. Viruses 2017, 9, 261. [Google Scholar] [CrossRef]
- Von Witzleben, A.; Wang, C.; Laban, S.; Savelyeva, N.; Ottensmeier, C.H. HNSCC: Tumour Antigens and Their Targeting by Immunotherapy. Cells 2020, 9, 2103. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Arbyn, M.; Turkot, M.H.; Wieszczy, P.; Miłosz, K.; Kamiński, M.F.; Didkowska, J.; Bidziński, M.; Olszewski, W.; Wielgoś, M.; et al. A Roadmap for a Comprehensive Control of Cervical Cancer in Poland: Integration of Available Solutions into Current Practice in Primary and Secondary Prevention. Eur. J. Cancer Prev. 2020, 29, 157–164. [Google Scholar] [CrossRef]
- Nowakowski, A.; Jach, R.; Szenborn, L.; Bidzinski, M.; Jackowska, T.; Kotarski, J.; Mastalerz-Migas, A.; Nitsch-Osuch, A.; Pinkas, J.; Sawicki, W.; et al. Recommendations of the Polish Society of Gynaecologists and Obstetricians, Polish Paediatric Society, Polish Society of Family Medicine, Polish Society of Vaccinology, Polish Society of Oncological Gynaecology and Polish Society of Colposcopy and Pathophysiology of the Uterine Cervix on Prophylactic Vaccinations against Infections with Human Papillomaviruses in Poland. Ginekol. Pol. 2023, 94, 759–767. [Google Scholar] [CrossRef]
- Basoya, S.; Anjankar, A. Cervical Cancer: Early Detection and Prevention in Reproductive Age Group. Cureus 2022, 14, e31312. [Google Scholar] [CrossRef]
- Charde, S.H.; Warbhe, R.A. Human Papillomavirus Prevention by Vaccination: A Review Article. Cureus 2022, 14, e30037. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.; Sundstrom, R.K. Cervical Intraepithelial Neoplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kornovski, Y.; Slavchev, S.; Kostov, S.; Ivanova, Y.; Yordanov, A. Precancerous Lesions of the Cervix—Aetiology, Classification, Diagnosis, Prevention. Oncol. Clin. Pract. 2021, 17, 271–276. [Google Scholar] [CrossRef]
- Tierney, K.E.; Roman, L.D.; Matsuo, K. Management of Cervical Dysplasia. In Handbook of Gynecology; Shoupe, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–11. ISBN 978-3-319-17002-2. [Google Scholar]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The Female Reproductive Tract Microbiotas, Inflammation, and Gynecological Conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef]
- Kumar, L.; Dwivedi, M.; Jain, N.; Shete, P.; Solanki, S.; Gupta, R.; Jain, A. The Female Reproductive Tract Microbiota: Friends and Foe. Life 2023, 13, 1313. [Google Scholar] [CrossRef]
- Plesniarski, A.; Siddik, A.B.; Su, R.-C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef]
- Moosa, Y.; Kwon, D.; De Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal Microbiota, Women’s Health, and Reproductive Outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V.; et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. [Google Scholar] [CrossRef]
- Molina, M.A.; Melchers, W.J.G.; Núñez-Samudio, V.; Landires, I. The Emerging Role of Lactobacillus Acidophilus in the Cervicovaginal Microenvironment. Lancet Microbe 2023, S2666524723003154. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Chen, W.-C.; Cheng, C.-M.; Shen, C.-J. Vaginal PH Value for Clinical Diagnosis and Treatment of Common Vaginitis. Diagnostics 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Andralojc, K.M.; Huynen, M.A.; Leenders, W.P.J.; Melchers, W.J.G. In-Depth Insights into Cervicovaginal Microbial Communities and HrHPV Infections Using High-Resolution Microbiome Profiling. NPJ Biofilms Microbiomes 2022, 8, 75. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- France, M.T.; Ma, B.; Gajer, P.; Brown, S.; Humphrys, M.S.; Holm, J.B.; Waetjen, L.E.; Brotman, R.M.; Ravel, J. VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition. Microbiome 2020, 8, 166. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Stoian, I.L.; Botezatu, A.; Fudulu, A.; Ilea, C.G.; Socolov, D.G. Exploring Microbiota Diversity in Cervical Lesion Progression and HPV Infection through 16S RRNA Gene Metagenomic Sequencing. J. Clin. Med. 2023, 12, 4979. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The Role of Viral Infection on Vaginal Microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-W.; Long, H.-Z.; Cheng, Y.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. From Microbiome to Inflammation: The Key Drivers of Cervical Cancer. Front. Microbiol. 2021, 12, 767931. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Andralojc, K.M.; Molina, M.A.; Qiu, M.; Spruijtenburg, B.; Rasing, M.; Pater, B.; Huynen, M.A.; Dutilh, B.E.; Ederveen, T.H.A.; Elmelik, D.; et al. Novel High-Resolution Targeted Sequencing of the Cervicovaginal Microbiome. BMC Biol. 2021, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Melchers, W.J.G.; Andralojc, K.M.; Leenders, W.P.J.; Huynen, M.A. Longitudinal Analysis on the Ecological Dynamics of the Cervicovaginal Microbiome in HrHPV Infection. Comput. Struct. Biotechnol. J. 2023, 21, 4424–4431. [Google Scholar] [CrossRef]
- Alizadehmohajer, N.; Shojaeifar, S.; Nedaeinia, R.; Esparvarinha, M.; Mohammadi, F.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Balouchi, A. Association between the Microbiota and Women’s Cancers—Cause or Consequences? Biomed. Pharmacother. 2020, 127, 110203. [Google Scholar] [CrossRef]
- Tsakmaklis, A.; Vehreschild, M.; Farowski, F.; Trommer, M.; Kohler, C.; Herter, J.; Marnitz, S. Changes in the Cervical Microbiota of Cervical Cancer Patients after Primary Radio-Chemotherapy. Int. J. Gynecol. Cancer 2020, 30, 1326–1330. [Google Scholar] [CrossRef]
- Hay, P.E.; Ugwumadu, A.; Chowns, J. Sex, Thrush and Bacterial Vaginosis. Int. J. STD AIDS 1997, 8, 603–608. [Google Scholar] [CrossRef]
- Dabee, S.; Passmore, J.-A.S.; Heffron, R.; Jaspan, H.B. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect. Immun. 2021, 89, e00487-20. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; Van De Wijgert, J.; Verstraelen, H. Vaginal Dysbiosis and the Risk of Human Papillomavirus and Cervical Cancer: Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- King, C.C.; Jamieson, D.J.; Wiener, J.; Cu-Uvin, S.; Klein, R.S.; Rompalo, A.M.; Shah, K.V.; Sobel, J.D. Bacterial Vaginosis and the Natural History of Human Papillomavirus. Infect. Dis. Obstet. Gynecol. 2011, 2011, 319460. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Lindner, B.; Krynicki, R.; Paziewska, A.; Nowakowski, A.; Bidzinski, M.; Ostrowski, J. Increased Diversity of a Cervical Microbiome Associates with Cervical Cancer. Front. Oncol. 2022, 12, 1005537. [Google Scholar] [CrossRef] [PubMed]
- Tsementzi, D.; Pena-Gonzalez, A.; Bai, J.; Hu, Y.; Patel, P.; Shelton, J.; Dolan, M.; Arluck, J.; Khanna, N.; Conrad, L.; et al. Comparison of Vaginal Microbiota in Gynecologic Cancer Patients Pre- and Post-radiation Therapy and Healthy Women. Cancer Med. 2020, 9, 3714–3724. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Han, M.; Wang, N.; Han, W.; Ban, M.; Sun, T.; Xu, J. Gut Microbes in Gynecologic Cancers: Causes or Biomarkers and Therapeutic Potential. Front. Oncol. 2022, 12, 902695. [Google Scholar] [CrossRef]
- Muls, A.; Andreyev, J.; Lalondrelle, S.; Taylor, A.; Norton, C.; Hart, A. Systematic Review: The Impact of Cancer Treatment on the Gut and Vaginal Microbiome in Women With a Gynecological Malignancy. Int. J. Gynecol. Cancer 2017, 27, 1550–1559. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhao, J.; Gong, L.; Zhang, Y.; Wang, X.; Yuan, Z. Altered Diversity and Composition of the Gut Microbiome in Patients with Cervical Cancer. AMB Expr. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Delgado Medrano, A.Y.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut Microbial Diversity and Genus-Level Differences Identified in Cervical Cancer Patients versus Healthy Controls. Gynecol. Oncol. 2019, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut Microbiome Diversity Is an Independent Predictor of Survival in Cervical Cancer Patients Receiving Chemoradiation. Commun. Biol. 2021, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-U.; Jung, D.-R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.-H. Dynamics of Fecal Microbiota with and without Invasive Cervical Cancer and Its Application in Early Diagnosis. Cancers 2020, 12, 3800. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role in Human Health and the Current Progress towards Its Clinical Application to Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Chang, L.; Qiu, L.; Lei, N.; Zhou, J.; Guo, R.; Gao, F.; Dong, S.; Chen, M.; Wu, F.; Qin, B. Characterization of Fecal Microbiota in Cervical Cancer Patients Associated with Tumor Stage and Prognosis. Front. Cell. Infect. Microbiol. 2023, 13, 1145950. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The Gut Microbiota Is Associated with Immune Cell Dynamics in Humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Li, C. The Role of TLRs in Cervical Cancer with HPV Infection: A Review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291–295. [Google Scholar] [CrossRef]
- Takeda, K. Toll-like Receptors in Innate Immunity. Int. Immunol. 2004, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Chauhan, A.; Raithatha, N.; Patel, P.; Desai, A.; Jain, N. Absence of Association between TLR4 Thr399Ile Polymorphism and Cervical Cancer Susceptibility. Meta Gene 2018, 17, 249–255. [Google Scholar] [CrossRef]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of Toll-like Receptors [TLR] 2 (−196 to −174 Del) and TLR 4 (Asp299Gly, Thr399Ile) in Cervical Cancer Susceptibility in North Indian Women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Zidi, S.; Sghaier, I.; Gazouani, E.; Mezlini, A.; Yacoubi-Loueslati, B. Evaluation of Toll-Like Receptors 2/3/4/9 Gene Polymorphisms in Cervical Cancer Evolution. Pathol. Oncol. Res. 2016, 22, 323–330. [Google Scholar] [CrossRef]
- de Moura, E.L.; dos Santos, I.F.; de Freitas, P.P.; da Silva, D.M.; dos Santos, A.C.M.; Lira Neto, A.B.; e Silva, A.C.P.; Barbosa, N.R.; Nascimento, C.A.; Balliano, T.L.; et al. Polymorphisms in Toll-like Receptors Genes Changes the Host’s Immune Response and Is Associated with Cervical Cancer. Immunobiology 2022, 227, 152187. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; DeCarlo, C.A.; Escott, N.; Zehbe, I.; Ulanova, M. Expression of Integrins and Toll-like Receptors in Cervical Cancer: Effect of Infectious Agents. Innate Immun. 2012, 18, 55–69. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, Y.; Shi, Y.; Xia, X.; Wang, S.; Duan, H. Expression and Functional Analysis of Toll-like Receptor 4 in Human Cervical Carcinoma. J. Membr. Biol. 2014, 247, 591–599. [Google Scholar] [CrossRef]
- Arany, I.; Tyring, S.K.; Stanley, M.A.; Tomai, M.A.; Miller, R.L.; Smith, M.H.; McDermott, D.J.; Slade, H.B. Enhancement of the Innate and Cellular Immune Response in Patients with Genital Warts Treated with Topical Imiquimod Cream 5%. Antivir. Res. 1999, 43, 55–63. [Google Scholar] [CrossRef]
- Borella, F.; Gallio, N.; Mangherini, L.; Cassoni, P.; Bertero, L.; Benedetto, C.; Preti, M. Recent Advances in Treating Female Genital Human Papillomavirus Related Neoplasms with Topical Imiquimod. J. Med. Virol. 2023, 95, e29238. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Sohn, K.-C.; Choi, D.-K.; Shi, G.; Hong, D.; Lee, H.-E.; Whang, K.U.; Lee, Y.H.; Im, M.; Lee, Y.; et al. Roles of TLR7 in Activation of NF-ΚB Signaling of Keratinocytes by Imiquimod. PLoS ONE 2013, 8, e77159. [Google Scholar] [CrossRef]
- Lin, C.-T.; Qiu, J.-T.; Wang, C.-J.; Chang, S.-D.; Tang, Y.-H.; Wu, P.-J.; Jung, S.-M.; Huang, C.-C.; Chou, H.-H.; Jao, M.-S.; et al. Topical Imiquimod Treatment for Human Papillomavirus Infection in Patients with and without Cervical/Vaginal Intraepithelial Neoplasia. Taiwan. J. Obstet. Gynecol. 2012, 51, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.O.; Possati-Resende, J.C.; Salcedo, M.P.; Schmeler, K.M.; Accorsi, G.S.; Fregnani, J.H.T.G.; Antoniazzi, M.; Pantano, N.P.; Santana, I.V.V.; Matsushita, G.M.; et al. Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Polterauer, S.; Natter, C.; Rahhal, J.; Hefler, L.; Tempfer, C.B.; Heinze, G.; Stary, G.; Reinthaller, A.; Speiser, P. Treatment of Cervical Intraepithelial Neoplasia With Topical Imiquimod: A Randomized Controlled Trial. Obstet. Gynecol. 2012, 120, 152–159. [Google Scholar] [CrossRef]
- Inayama, Y.; Yamanishi, Y.; Nakatani, E.; Aratake, J.; Sasagasako, N.; Yamada, K.; Gou, R.; Kawamura, A.; Yamanishi, M.; Kosaka, K. Imiquimod for Vaginal Intraepithelial Neoplasia 2–3: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2021, 160, 140–147. [Google Scholar] [CrossRef]
- Inayama, Y.; Takamatsu, S.; Hamanishi, J.; Mizuno, K.; Horinouchi, N.; Yamanoi, K.; Taki, M.; Murakami, R.; Yamaguchi, K.; Kosaka, K.; et al. Imiquimod for Cervical and Vaginal Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2023, 142, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and Targeted Therapy in the Management of Cervical Cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- Mitra, A.; Gultekin, M.; Burney Ellis, L.; Bizzarri, N.; Bowden, S.; Taumberger, N.; Bracic, T.; Vieira-Baptista, P.; Sehouli, J.; Kyrgiou, M. Genital Tract Microbiota Composition Profiles and Use of Prebiotics and Probiotics in Gynaecological Cancer Prevention: Review of the Current Evidence, the European Society of Gynaecological Oncology Prevention Committee Statement. Lancet Microbe 2023, 2023, S2666524723002574. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Tajadadi-Ebrahimi, M.; Kolahdooz, F.; Mazouchi, M.; Hadaegh, H.; Jamal, A.-S.; Mazroii, N.; Asemi, S.; Asemi, Z. The Consumption of Synbiotic Bread Containing Lactobacillus Sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2016, 35, 506–513. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Criscuolo, A.A.; Sesti, F.; Piccione, E.; Mancino, P.; Belloni, E.; Gullo, C.; Ciotti, M. Therapeutic Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Women with Cervical Uterine High-Risk HPV Infection: A Retrospective Observational Study. Adv. Ther. 2021, 38, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lavitola, G.; Della Corte, L.; De Rosa, N.; Nappi, C.; Bifulco, G. Effects on Vaginal Microbiota Restoration and Cervical Epithelialization in Positive HPV Patients Undergoing Vaginal Treatment with Carboxy-Methyl-Beta-Glucan. BioMed Res. Int. 2020, 2020, 5476389. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; López, A.C.; González, S.P.; Palacios, S.; Dexeus, D.; Centeno-Mediavilla, C.; Coronado, P.; de la Fuente, J.; López, J.A.; Vanrell, C.; et al. Efficacy of a Coriolus Versicolor–Based Vaginal Gel in Women With Human Papillomavirus–Dependent Cervical Lesions: The PALOMA Study. J. Low. Genit. Tract. Dis. 2021, 25, 130–136. [Google Scholar] [CrossRef]
- Laccetta, G.; Carrone, A.; Burratti, M.; Mancino, P. Effect of the Treatment with β-Glucan in Women with Cervical Cytologic Report of Atypical Squamous Cells of Undetermined Significance (ASCUS) and Low-Grade Squamous Intraepithelial Lesions (L-SIL). Minerva Ginecol. 2015, 67, 113–120. [Google Scholar]
- Stentella, P.; Biamonti, A.; Carraro, C.; Inghirami, P.; Mancino, P.; Pietrangeli, D.; Votano, S.; Lazzari, P.; De Medici, C. Efficacy of Carboxymethyl Beta-Glucan in Cervical Intraepithelial Neoplasia: A Retrospective, Case-Control Study. Minerva Obs. Gynecol. 2017, 69, 425–430. [Google Scholar] [CrossRef]
- Gil-Antuñano, S.P.; Serrano Cogollor, L.; López Díaz, A.C.; González Rodríguez, S.P.; Dexeus Carter, D.; Centeno Mediavilla, C.; Coronado Martín, P.; de la Fuente Valero, J.; López Fernández, J.A.; Vanrell Barbat, C.; et al. Efficacy of a Coriolusversicolor-Based Vaginal Gel in Human Papillomavirus-Positive Women Older Than 40 Years: A Sub-Analysis of PALOMA Study. J. Pers. Med. 2022, 12, 1559. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agents Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Di Pierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial. Minerva Obs. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef]
- Smith, J.A.; Gaikwad, A.A.; Mathew, L.; Rech, B.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. AHCC® Supplementation to Support Immune Function to Clear Persistent Human Papillomavirus Infections. Front. Oncol. 2022, 12, 881902. [Google Scholar] [CrossRef] [PubMed]
- dE Vet, H.C.W.; Knipschild, P.G.; Willebrand, D.; Schouten, H.J.A.; Sturmans, F. The Effect of Beta-Carotene on the Regression and Progression of Cervical Dysplasia: A Clinical Experiment. J. Clin. Epidemiol. 1991, 44, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, L.; Sukhikh, G.; Kiselev, V.; Paltsev, M.; Drukh, V.; Kuznetsov, I.; Muyzhnek, E.; Apolikhina, I.; Andrianova, E. Double-Blind Randomized Placebo-Controlled Multicenter Clinical Trial (Phase IIa) on Diindolylmethane’s Efficacy and Safety in the Treatment of CIN: Implications for Cervical Cancer Prevention. EPMA J. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.-K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective Effects of Green Tea Extracts (Polyphenon E and EGCG) on Human Cervical Lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.C.; Crowley-Nowick, P.; Bradlow, H.L.; Sepkovic, D.W.; Schmidt-Grimminger, D.; Howell, P.; Mayeaux, E.J.; Tucker, A.; Turbat-Herrera, E.A.; Mathis, J.M. Placebo-Controlled Trial of Indole-3-Carbinol in the Treatment of CIN. Gynecol. Oncol. 2000, 78, 123–129. [Google Scholar] [CrossRef]
- Ayatollahi, H.; Rajabi, E.; Yekta, Z.; Jalali, Z. Efficacy of Oral Zinc Sulfate Supplementation on Clearance of Cervical Human Papillomavirus (HPV); A Randomized Controlled Clinical Trial. Asian Pac. J. Cancer Prev. 2022, 23, 1285–1290. [Google Scholar] [CrossRef]
- Walker, H.; Burrell, M.; Flatley, J.; Powers, H. A Metabolite Profiling Method for Diagnosis of Precancerous Cervical Lesions and HPV Persistence. Bioanalysis 2017, 9, 601–608. [Google Scholar] [CrossRef]
- Pappa, K.I.; Daskalakis, G.; Anagnou, N.P. Metabolic Rewiring Is Associated with HPV-Specific Profiles in Cervical Cancer Cell Lines. Sci. Rep. 2021, 11, 17718. [Google Scholar] [CrossRef]
- Porcari, A.M.; Negrão, F.; Tripodi, G.L.; Pitta, D.R.; Campos, E.A.; Montis, D.M.; Martins, A.M.A.; Eberlin, M.N.; Derchain, S.F.M. Molecular Signatures of High-Grade Cervical Lesions. Front. Oncol. 2018, 8, 99. [Google Scholar] [CrossRef]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Fang, B.; Li, Q.; Wan, Z.; OuYang, Z.; Zhang, Q. Exploring the Association Between Cervical Microbiota and HR-HPV Infection Based on 16S RRNA Gene and Metagenomic Sequencing. Front. Cell Infect. Microbiol. 2022, 12, 922554. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujii, T.; Kukimoto, I.; Nomura, H.; Kawasaki, R.; Nishio, E.; Ichikawa, R.; Tsukamoto, T.; Iwata, A. Changes to the Cervicovaginal Microbiota and Cervical Cytokine Profile Following Surgery for Cervical Intraepithelial Neoplasia. Sci. Rep. 2021, 11, 2156. [Google Scholar] [CrossRef]
- Kaelin, E.A.; Skidmore, P.T.; Łaniewski, P.; Holland, L.A.; Chase, D.M.; Herbst-Kralovetz, M.M.; Lim, E.S. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. mSystems 2022, 7, e00064-22. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Yang, Y.; Liao, H. Changes in the Cervicovaginal Microbiota Composition of HPV16-infected Patients after Clinical Treatment. Cancer Med. 2022, 11, 5037–5049. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, B.-S.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Park, S.-Y.; Hong, K.-M.; Kim, H.-K.; Kim, M.K. The Association of Uterine Cervical Microbiota with an Increased Risk for Cervical Intraepithelial Neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef]
- Ritu, W.; Enqi, W.; Zheng, S.; Wang, J.; Ling, Y.; Wang, Y. Evaluation of the Associations Between Cervical Microbiota and HPV Infection, Clearance, and Persistence in Cytologically Normal Women. Cancer Prev. Res. 2019, 12, 43–56. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Wiik, J.; Sengpiel, V.; Kyrgiou, M.; Nilsson, S.; Mitra, A.; Tanbo, T.; Monceyron Jonassen, C.; Møller Tannæs, T.; Sjøborg, K. Cervical Microbiota in Women with Cervical Intra-Epithelial Neoplasia, Prior to and after Local Excisional Treatment, a Norwegian Cohort Study. BMC Women’s Health 2019, 19, 30. [Google Scholar] [CrossRef]
- Wu, S.; Ding, X.; Kong, Y.; Acharya, S.; Wu, H.; Huang, C.; Liang, Y.; Nong, X.; Chen, H. The Feature of Cervical Microbiota Associated with the Progression of Cervical Cancer among Reproductive Females. Gynecol. Oncol. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhang, W.; Zhang, Z.; Fu, Y.; Li, Y.; Wang, X.; Li, L.; Meng, Y. Characteristics of the Cervicovaginal Microenvironment in Childbearing-Age Women with Different Degrees of Cervical Lesions and HR-HPV Positivity. Pol. J. Microbiol. 2021, 70, 489–500. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Gao, W.; Pan, Y.; Gao, Y.; Shen, J.; Xiong, H. The Direct and Indirect Association of Cervical Microbiota with the Risk of Cervical Intraepithelial Neoplasia. Cancer Med. 2018, 7, 2172–2179. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Lu, Y.; Cai, Q.; Liu, H.; Xu, C. Cervical Microbiome Is Altered in Cervical Intraepithelial Neoplasia after Loop Electrosurgical Excision Procedure in China. Sci. Rep. 2018, 8, 4923. [Google Scholar] [CrossRef]
- Wei, L.Q.; Cheong, I.H.; Yang, G.H.; Li, X.G.; Kozlakidis, Z.; Ding, L.; Liu, N.N.; Wang, H. The Application of High-Throughput Technologies for the Study of Microbiome and Cancer. Front. Genet. 2021, 12, 699793. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef]
- Winand, R.; Bogaerts, B.; Hoffman, S.; Lefevre, L.; Delvoye, M.; Van Braekel, J.; Fu, Q.; Roosens, N.H.; De Keersmaecker, S.C.; Vanneste, K. Targeting the 16S RRNA Gene for Bacterial Identification in Complex Mixed Samples: Comparative Evaluation of Second (Illumina) and Third (Oxford Nanopore Technologies) Generation Sequencing Technologies. Int. J. Mol. Sci. 2019, 21, 298. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S RRNA Gene Sequence Analysis for Identification of Bacteria on Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Smith, B.C.; McAndrew, T.; Chen, Z.; Harari, A.; Barris, D.M.; Viswanathan, S.; Rodriguez, A.C.; Castle, P.; Herrero, R.; Schiffman, M.; et al. The Cervical Microbiome over 7 Years and a Comparison of Methodologies for Its Characterization. PLoS ONE 2012, 7, e40425. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Curty, G.; Costa, R.L.; Siqueira, J.D.; Meyrelles, A.I.; Machado, E.S.; Soares, E.A.; Soares, M.A. Analysis of the Cervical Microbiome and Potential Biomarkers from Postpartum HIV-Positive Women Displaying Cervical Intraepithelial Lesions. Sci. Rep. 2017, 7, 17364. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Li, F.; Zhao, J.; Wan, X.; Wang, K. Cervicovaginal Microbiota Composition Correlates with the Acquisition of High-Risk Human Papillomavirus Types. Int. J. Cancer 2018, 143, 621–634. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Seo, S.S.; Kwon, M.; Lee, J.K.; Kim, M.K. Association of Cervical Microbial Community with Persistence, Clearance and Negativity of Human Papillomavirus in Korean Women: A Longitudinal Study. Sci. Rep. 2018, 8, 15479. [Google Scholar] [CrossRef]
- Ravilla, R.; Coleman, H.N.; Chow, C.-E.; Chan, L.; Fuhrman, B.J.; Greenfield, W.W.; Robeson, M.S.; Iverson, K.; Spencer, H.; Nakagawa, M. Cervical Microbiome and Response to a Human Papillomavirus Therapeutic Vaccine for Treating High-Grade Cervical Squamous Intraepithelial Lesion. Integr. Cancer Ther. 2019, 18, 153473541989306. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.-L.; Mbulawa, Z.Z.A.; Coetzee, D.; Meiring, T.L. The Cervical Microbiota in Reproductive-Age South African Women with and without Human Papillomavirus Infection. Papillomavirus Res. 2019, 7, 154–163. [Google Scholar] [CrossRef]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal Microbiome and Natural History of HPV in a Longitudinal Study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Zhang, D.; Zong, X.; Bai, H.; Bi, H.; Liu, Z. Distinction between Vaginal and Cervical Microbiota in High-Risk Human Papilloma Virus-Infected Women in China. BMC Microbiol. 2021, 21, 90. [Google Scholar] [CrossRef]
- Sasivimolrattana, T.; Chantratita, W.; Sensorn, I.; Chaiwongkot, A.; Oranratanaphan, S.; Bhattarakosol, P.; Bhattarakosol, P. Cervical Microbiome in Women Infected with HPV16 and High-Risk HPVs. Int. J. Environ. Res. Public. Health 2022, 19, 14716. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, H.; Yuan, L.; Chen, X.; Huang, X.; Wang, K.; Li, Z. Vaginal Microbiota and HPV Clearance: A Longitudinal Study. Front. Oncol. 2022, 12, 955150. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Xiao, W.-Y.; Fang, J.-F.; Dong, Y.-H.; Ye, K.-F.; He, M.-P.; Wang, Y.-S.; Li, X.; Zhao, Z.-M.; Yuan, T.; et al. Genital Microbiota of Women From Six Ethnic Groups With and Without Human Papillomavirus Infection in Shangri-La, China. Front. Cell. Infect. Microbiol. 2022, 12, 935068. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Quan, L.; Yang, W.; Lang, J.; Tian, G.; Meng, B. Research of Cervical Microbiota Alterations with Human Papillomavirus Infection Status and Women Age in Sanmenxia Area of China. Front. Microbiol. 2022, 13, 1004664. [Google Scholar] [CrossRef]
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal Microbiota Significantly Changed for HPV-Positive Women with High-Grade Squamous Intraepithelial Lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875. [Google Scholar] [CrossRef]
- Vikramdeo, K.S.; Anand, S.; Pierce, J.Y.; Singh, A.P.; Singh, S.; Dasgupta, S. Distribution of Microbiota in Cervical Preneoplasia of Racially Disparate Populations. BMC Cancer 2022, 22, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Liang, Y.; Wei, L.; Zhang, W.; Li, L. Observation of the Cervical Microbiome in the Progression of Cervical Intraepithelial Neoplasia. BMC Cancer 2022, 22, 362. [Google Scholar] [CrossRef]
- Liu, H.; Liang, H.; Li, D.; Wang, M.; Li, Y. Association of Cervical Dysbacteriosis, HPV Oncogene Expression, and Cervical Lesion Progression. Microbiol. Spectr. 2023, 10, e00151-22. [Google Scholar] [CrossRef]
- Vargas-Robles, D.; Romaguera, J.; Alvarado-Velez, I.; Tosado-Rodríguez, E.; Dominicci-Maura, A.; Sanchez, M.; Wiggin, K.J.; Martinez-Ferrer, M.; Gilbert, J.A.; Forney, L.J.; et al. The Cervical Microbiota of Hispanics Living in Puerto Rico Is Nonoptimal Regardless of HPV Status. mSystems 2023, 8, e00357-23. [Google Scholar] [CrossRef]
- Teka, B.; Yoshida-Court, K.; Firdawoke, E.; Chanyalew, Z.; Gizaw, M.; Addissie, A.; Mihret, A.; Colbert, L.E.; Napravnik, T.C.; El Alam, M.B.; et al. Cervicovaginal Microbiota Profiles in Precancerous Lesions and Cervical Cancer among Ethiopian Women. Microorganisms 2023, 11, 833. [Google Scholar] [CrossRef]
- Rokos, T.; Holubekova, V.; Kolkova, Z.; Hornakova, A.; Pribulova, T.; Kozubik, E.; Biringer, K.; Kudela, E. Is the Physiological Composition of the Vaginal Microbiome Altered in High-Risk HPV Infection of the Uterine Cervix? Viruses 2022, 14, 2130. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated with Increased Vaginal Microbiome Diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, B.; Jie, Z.; Songben, Q.; Juan, C.; Lei, Z.; Mu, X. Cervicovaginal Microbiota Dysbiosis Correlates with HPV Persistent Infection. Microb. Pathog. 2021, 152, 104617. [Google Scholar] [CrossRef]
- Chorna, N.; Romaguera, J.; Godoy-Vitorino, F. Cervicovaginal Microbiome and Urine Metabolome Paired Analysis Reveals Niche Partitioning of the Microbiota in Patients with Human Papilloma Virus Infections. Metabolites 2020, 10, 36. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The Vaginal Microbiota Associates with the Regression of Untreated Cervical Intraepithelial Neoplasia 2 Lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef]
- Berggrund, M.; Gustavsson, I.; Aarnio, R.; Lindberg, J.H.; Sanner, K.; Wikström, I.; Enroth, S.; Bunikis, I.; Olovsson, M.; Gyllensten, U. Temporal Changes in the Vaginal Microbiota in Self-Samples and Its Association with Persistent HPV16 Infection and CIN2+. Virol. J. 2020, 17, 147. [Google Scholar] [CrossRef]
- Liu, J.; Luo, M.; Zhang, Y.; Cao, G.; Wang, S. Association of High-Risk Human Papillomavirus Infection Duration and Cervical Lesions with Vaginal Microbiota Composition. Ann. Transl. Med. 2020, 8, 1161. [Google Scholar] [CrossRef]


| Author, Year, Country | Study Aim | Groups | Material and Detection Method | Results | Changes in Microbiota Abundance | Conclusions | |
|---|---|---|---|---|---|---|---|
| Cases N, (Age) | Controls N, (Age) | ||||||
| Smith et al., 2012, USA [124] | Evaluation of methodological variables of cervical microbiome analysis performance and stability of cervical microbiome collected annually over a period of 5–7 years. | 10 HPV+ women in the Natural History Study of HPV in Guanacaste, Costa Rica | Cervical swabs V6 and V6–V9 regions of the 16S rRNA gene; Sanger, Roche 454, and Illumina HiSeq 2000 | Ns. differences in age, disease stage, HPV subtype, microbiota community types, and diversity between the HPV-cleared and HPV-uncleared groups. Women with depleted enterococcus ASV_62 and enriched L. iners at baseline less likely to achieve HPV clearance at month 12. A negative association between high L. Iners abundance and HPV clearance in non-operative treatment patients but not in those who received operative treatment. | Increase: HPV+: Lactobacillus, Gardnerella | The Roche 454 and Illumina sequencing yielded different community type assignments for certain samples. The primary transition between community types was mainly attributed to a shift between L. iners and G. vaginalis, which were overwhelmingly dominant. | |
| Oh et al., 2015, Korea [111] | To investigate the connection between CIN and the CM identified through pyrosequencing. | 70 with CIN (18–65) | 50 controls (18–65) | Cervical swabs Pyrosequencing V1–V3 regions of 16S rRNA Roche/454 GS Junior system | TheIU number was higher in HPV− than HPV+ women. | Increase: Bacteroidetes, Actinobacteria, Tenericutes, Proteobacteria higher in HPV+ women; A. vaginae, P. bivia, L. fornicalis, P. Poae, and G. vaginalis Decrease: L. iners and L. crispatus | The presence of bacterial dysbiosis, characterized by an abundance of A. vaginae, G. vaginalis, and L. iners, along with a scarcity of L. crispatus, in combination with oncogenic HPV, may be a risk factor for cervical lesion. |
| Piyathilake et al., 2016, USA [112] | To investigate the relationship between the CVM and CIN-2+ in women with well-defined HPV infection and confirmed CIN lesions, considering other risk factors as well. | 340 CIN-2 HPV+ (19–50) | 90 CIN-1 HPV+ (19–50) | Cervical mucus samples V4 region of the 16S rRNA gene sequenced using Illumina MiSeq | Of the HPV groups, six phyla, Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, Fusobacteria, and Tenericutes, were dominant. Proteobacteria and Firmicutes were the predominant phyla in most women. | Increase: CIN-2 HPV+: L. iners and unclassified Lactobacillus spp., Lactobacillaceae, Lactobacillus, L. reuteri, and several sub-genus level Lactobacillus OTUs; Bacteroidaceae, Porphyromonadacae, and Coxiellaceae; genera including Bacteroides, Parabacteroides, Rickettsiella, and RFN20 | An association between microbiome diversity and CIN severity or oxidative DNA damage was not observed. However, suggestive evidence indicated that CIN-2+ in women infected with hrHPVs might be linked to a cervical microbiome predominantly composed of Lactobacillus and L. iners. |
| Audirac-Chalifour et al., 2016, Mexico [106] | To examine the association between CM diversity and composition based on the histopathological diagnosis of each stage of the natural history of CC and the cervical expression levels of IL-4, IL-6, IL-10, TGF-β1, TNF-α, and IFN-γ mRNA. | 124 HPV+ (22–61) | 81 HPV− (22–61) | Cervical swabs V3–V4 variable regions from 16S rRNA Roche 454, Genome Sequencer Titanium | L. iners was the most prevalent species in the cervix among HPV-infected women without lesions. | Increase: HPV+: P. oleovorans, L. iners, Sneathia spp., S. satelles, M. elsdenii, HPV−: L. crispatus, G. vaginalis Decrease: HPV+: G. vaginalis HPV−: L. iners | It is suggested that the CM may be involved in CC pathology. During the development of SIL and CC, some members of the CM could potentially act as modifiers of the cervical microenvironment’s cytokine profile. Accumulating evidence indicates a major role of the microbiota in the immune system modulation of the female genital tract. |
| Di Paola et al., 2017, Italy [125] | To describe the CSTs linked to HPV-persistence. | 27 Clearance—hrHPV infection cleared after one year with no DNA evidence. 28 Persistence—hrHPV infection persisted. (26–64) | 17 age-matched HPV− women. (26–64) | Cervicovaginal samples V3–V5 hypervariable regions of 16S rRNA gene Roche 454, GS FLX+ system | Lactobacillus was the most abundant genus in CVM. Biodiversity higher in HPV+ group, especially in persistence group vs. control group. | Increase: L. crispatus—dominant Lactobacillus species in control and clearance groups. Persistence group—higher Atopobium levels. Clearance group—mix of aerobic and anaerobic bacteria (Pseudomonas, Brevibacterium, Peptostreptococcus, Enterococcus, Streptococcus, Propionibacterium, Bifidobacterium, Shigella). Decrease: The persistence group—lower presence of the Faecalibacterium compared to the clearance group | Persistence group—low alpha diversity, limited bacterial genera linked to viral persistence. A. vaginae abundant, may disrupt epithelial barriers, increasing HPV infection risk. Early CVM characterization identifies high-risk women and informs therapeutic strategies. |
| Curty et al., 2017, Brazil [126] | To report the initial data on the CM of HIV+ women in the postpartum period. Specific microbiota species as indicators detecting alterations in the cervical microenvironment linked to cervical lesions. | 80 women in the Program for HIV-infected Pregnant Women at the Federal University of Rio Janeiro (UFRJ). 25 subjects had samples available at time points of 6 and 12 months. (17–44) | 80 women (single timepoint); 26 women samples at the 6-month postpartum time point. In total, 105 individual samples. (17–44) | Cervical cytobrushes V3–V6 regions of 16S rRNA gene Illumina HiSeq 2500 system | The CM of HIV+ women during the postpartum period remained stable, exhibiting a diverse range of bacteria without a dominant presence of L.crispatus. Three bacterial genera (Moryella, Schlegella, and Gardnerella) were associated with cervical lesions. Poor knowledge of the functional roles of these bacteria in CM homeostasis and their influence on the development of CC. | Increase: One in high abundance (Gardnerella) Decrease: Bacterial genera in low abundance (Bifidobacterium, Moryella, Schlegella, and Aerococcus) | The interaction between the CM, local environment, and immune system is intricate and crucial for maintaining cervical homeostasis. Distinct species within the microbiota function as indicators, detecting changes in the cervical microenvironment, and potentially contributing to its modulation or being influenced by it, leading to either a healthy or diseased state. |
| Huang et al., 2018, China [127] | To explore the relationship between community composition and single hrHPV-type infection and the relationship between the differentially present microbial species and their effect on hrHPV-type acquisition. | Infected with HPV16, HPV52, and HPV58; both LSIL and HSIL (18–70) | 41 healthy women HPV− (18–70) | Cervical cytobrushes V4–V5 regions of 16S rRNA gene, Illumina MiSeq platform | A specific microbial pattern in each hrHPV type was identified, and the crucial microbial species associated with them were characterized. | Increase: HPV16: Oribacterium, Lachnobacterium, Thermus HPV52: Motilibacter HPV58: Litorilinea, Paludibaculum HPV+: Firmicutes, Actinobacteria, Fusobacteria; P. aquiterrae, E. brevis, M. indicum, A. guillouiae, A. citratiphilum, L. kribbensis HPV−: Proteobacteria, B. stagnalis Decrease: HPV58: L. iners HPV+: Proteobacteria, B. stagnalis, B. territorii, P. mucidolens HPV−: Firmicutes, Actinobacteria, Fusobacteria | The acquisition of hrHPV does not seem to be influenced by a common CVM group, but rather by specific pathogenic agents unique to each SIL, irrespective of their abundance. |
| Zhang et al., 2018, Chiny [119] | To investigate the changes in the cervical microbiome after LEEP treatment. | 26 HPV+ patients who underwent LEEP for CIN-2 or CIN-3 (25–68) | Cervical swabs V3–V4 hyper-variable regions of the 16S rRNA gene sequenced using Illumina MiSeq | CiRNAseq and 16S rRNA-seq showed similar efficiency in identifying and quantifying microbes. They were in agreement for 81% of the analyzed genera (31 out of 38), demonstrating the high specificity and sensitivity of CiRNAseq at the genus level. | Increase: 3 months after LEEP: L. iners; Erysipelotrichaceae and Coriobacteriaceae;Before LEEP: Bifidobacteriaceae, Lachnospiraceae, Leptotrichiaceae, Peptostreptococcaceae, S. amnii, Collinsella, Veillonella, Clostridia, Prevotella, and unclassified genus belonging to Lachnospiraceae. | In patients with CIN-2/3, LEEP treatment leads to changes in the cervical microbiome. However, LEEP alone is insufficient to fully restore a healthy cervical bacterial community. | |
| Arokiyaraj et al., 2018, Korea [128] | To identify cervical microbes associated with HPV negativity, HPV clearance, and HPV persistence. To assess the longitudinal connections between these microbes and HPV infection dynamics among Korean women. | HPV clearance (42 samples, 15 subjects) HPV persistence (44 samples, 16 subjects) (18–65) | HPV negativity (21 samples, 10 subjects) (18–65) | Cervical cytobrushes V3–V5 hypervariable regions of 16S rRNA gene, Roche 454 GS-FLX plus | Higher diversity was observed in HPV-persistence women compared to HPV− women. | Increase: HPV clearance: the strongest associations with E. eligens, G. vaginalis, and U. urealyticum. HPV persistence: was strongly associated with L. johnsonii. HPV+: the highest abundance of A. vaginae. Decrease: L. crispatus was mainly dominated by the HPV− group | It is suggested that the presence and prevalence of a specific cervical microbiome are factors involved in HPV dynamics. The strongest associations with HPV persistence were observed in women with high proportions of L. johnsonii, Haemophilus (genus), and Mycoplasmataceae (family). |
| Zhang et al., 2018, China [118] | To examine the relationships between microbiotas and the severity of CIN, both directly and indirectly. | 126 women with CIN-1− (normal cytology and CIN-1); 40 with CIN-2+ (CIN-2 and 3). | Biopsy specimens V3–V6 regions of 16S rRNA gene, Illumina HiSeq 2500 platform | Highly sensitive PCR primer set (SPF1/GP6+) used to detect HPV DNA by amplifying a 184-bp fragment of the L1 open reading frame may still underestimate the proportion of certain HPV infections. | Increase: HPV+: S. agalactiae, B. fragilis, P. stutzeri, and P. anaerobius. CIN-2+: L. crispatus, S. agalactiae, B. fragilis, and C. ureolyticus. Decrease: HPV−: L. delbrueckii CIN-2-: P. damselae, L. jensenii, and A. vaginae | Given the small sample size and the sampling site limited to the location of CIN rather than the entire cervix, the prevalence of HPV infection in our participants may have been underestimated. Considering the limited number of HPV+ samples, the intention was to include a larger number of individuals in future studies to validate our findings. | |
| Ravilla et al., 2019, USA [129] | To assess the potential impact of the cervical microbiome on vaccine response and explore the determinants of the cervical microbiome composition in women diagnosed with high-grade squamous intraepithelial lesions. | 31 patients who received vaccination, with biopsy-proven CIN-2/3 (22–49) | Cervical brushes Fragmented and labeled with biotin amplicons of 16S rRNA, hybridized to the PhyloChip Array (version G4) | Ns. difference in HPV contig richness was found by genital inflammation status or microbiome profile. | Between HPV16+ and HPV16- samples, notable variations in beta diversity were found. A total of 15 eOTUs displayed significant differences in their abundances. | Certain bacterial taxa, including Caldithrix, Nitrospirae, and Prevotella, might influence the response to the HPV therapeutic vaccine. However, vaccination did not seem to impact the composition of the cervical microbiome. Race and HPV16 infection seemed to have an influence on the beta diversity of the cervical microbiome. | |
| Onywera et al., 2019, South Africa [130] | To investigate the composition and diversity of CM in reproductive-age black South African women and explore their connections with HPV infections. | 37 women (18–65) | 50 women (18–65) | Cervical swabs V3–V4 regions of 16S rRNA gene, Illumina MiSeq platform | A total of 28 bacterial taxa were found to exhibit differential abundance between the CM of HPV− and HPV+ women. Neither Lactobacillus nor species within this genus were found to be differentially abundant between women with and without HPV or hrHPV infections. | Increase: In comparison to those with lrHPV or no HPV-infection, women with hrHPV displayed significantly higher relative abundances of Aerococcaceae, Pseudomonadaceae, and Bifidobacteriaceae. Furthermore, Gardnerella, Sneathia, and Atopobium were also found to have higher relative abundances in hrHPV-infected women compared to those with lrHPV or HPV−. Decrease: Campylobacter, Haemophilus, and Pseudomonas. | To date, the association between prevalent HPV and CM in a black South African cohort has been examined for the first time. Further investigations into the role of the cervical and vaginal microbiome in HPV/hrHPV infections are warranted. |
| Ritu et al., 2019, China [113] | To investigate the connections between CM and various HPV infection statuses in women with normal cytology; analysis of the variations in CM linked to the acquisition, persistence, and clearance of different HPV genotypes through a one-year follow-up period. | 90 HPV+ (27–65) | 43 HPV− (27–65) | Cervical swabs 16S rDNA sequencing with Illumina Hiseq 2500 platform. | Several taxa that could distinguish baseline HPV positivity and predict the acquisition, persistence, or clearance of HPV within a one-year period was discovered. No significant difference in evenness diversity was observed among different HPV infection statuses. | HPV+ women compared to HPV− exhibited higher richness influenced by the abundance of genera other than Lactobacillus, including Acinetobacter, Burkholderia, Campylobacter, Pseudomonas, Corynebacterium, Halorubrum, and Halorientalis. This richness was found to have the strongest correlation with evenness diversity. | Specific compositions of the CM associated with distinct HPV infection statuses could serve as a biomarker to identify women at risk of persistent HPV infection. Further investigations into the mechanisms underlying these associations may provide valuable insights for developing new therapeutic strategies that modify the microbiota of the reproductive tract to enhance HPV infection clearance. |
| Usyk et al., 2020, Costa Rica [131] | To investigate the impact of the CVM on the natural history of incident hrHPV infections, focusing on three key aspects: 1. Advancement to cervical precancerous stages; 2. Duration of viral presence in the body; 3. Elimination of the virus from the body (viral clearance). | 273 women recruited at first clinical visit (V1)—HPV testing. 266 in follow up examination, at a subsequent visit (V2), meeting criteria for persistence (having the same HPV type at least 305 days after V1), progression (closest visit before diagnosis of CIN2+), or clearance (following visit negative for that type). | Cervical brushes V4 variable region of the 16S rRNA gene, Illumina MiSeq platform | Increase: L. iners linked to the clearance of newly acquired hrHPV infections (V1); Gardnerella dominant biomarker associated with hrHPV progression. | Gardnerella affects the CVM balance, influencing hrHPV progression to precancer. Positive association between Gardnerella at V1 and CIN2+ progression was mediated by the increased CVM diversity observed at V2. | ||
| Andralojc et al., 2021, The Netherlands [41] | To evaluate the potential of the CVM-specific CiRNAseq assay, validate the technique’s resolution, specificity, and performance in vitro using mock samples, and profile the CVM in a cohort of cervical smears from women with or without hrHPV-associated cervical abnormalities. | 46 HPV− women: RNA isolation + CiRNAseq 46 HPV+ women with CIN-2+: RNA isolation + CiRNAseq | 10 HPV+ women—DNA isolation + CiRNAseq | Cervical smears CiRNAseq, Illumina NextSeq platform | The top 10 virus genera included: the most dominant Alphapapillomavirus (includes HPV), Betatomopoxvirus, Betabaculovirus, Simplexvirus, Cafeteriavirus, Coccolithovirus, Mimivirus, Betaretrovirus, Ichnovirus, and Alphabaculovirus. HPV16, 32, and 53 were the most prevalent. The HPV-dominated group: 47.62% CIN-1 and 42.86% CIN-2/3 samples; the non-HPV-dominated group: 52.38% CIN-1 and 57.14% CIN-2/3 samples. | Increase: HPV−: L. acidophilus, L. crispatus, L. jensenii, L. psittaci, L. ultunensis, L. vaginalis HPV+: A. vaginae, D. micraerophilus, G. vaginalis, S. amnii, S. sanguinegens, L. iners and Prevotella species: P. amnii, P. buccalis, P. timonensis Decrease: After 3 months of LEEP treatment: cervical microbial diversity, L. amnionii, and Clostridium sensu stricto | CiRNAseq revealed that the the CVM transitions from a healthy Lactobacillus-dominated state (CST I) to an anaerobic-diverse state (CST IV) during persistent hrHPV infection. CiRNAseq proves to be a highly promising technology with its high-resolution and specificity for high-throughput sequencing, making it an intriguing tool for exploring the role of CVM in both health and disease. |
| Wu et al., 2021, China [116] | To examine the cervical microbiome characteristics in reproductive-age women during the transition from SIL to CC. | 13 women with CC, 31 HSIL, 10 LSIL, 12 HPV+ (NH) (18–52) | 28 healthy controls (NN) (18–52) | Cervical swabs V4 region of 16S rRNA gene, Illumina NovoSeq6000 | CC group had the highest community diversity of CM. | Increase: Prevotella, Megasphaera Decrease: Lactobacillus | As the lesions progressed, there was a noticeable upward trend in species diversity. |
| Zhai et al., 2021, China [117] | Examining the CVM in women of childbearing age with different degrees of cervical lesions and hrHPV positivity. | 29 hrHPV+ 32 LSIL 40 HSIL 38 CC (30–50) | 29 HPV− women (30–50) | Cervical swabs V3–V4 regions of 16S rRNA gene, IonS5TMXL platform | In the healthy group, Prevotella suppressed the abundance of Lactobacillus. In the disease groups, Prevotella promoted the abundance of Gardnerella. | Increase: Actinobacteria, Gardnerella, and Prevotella Decrease: Firmicutes, Lactobacillus, Ignatzschineria, and Streptococcus | The healthy group: a strongest association with the genera Lactobacillus and Ignatzschineria. The disease groups were most closely related to the genera Gardnerella and Prevotella. A vaginal environment with low abundances of Lactobacillus and Ignatzschineria might facilitate the progression of lesions into cancer. |
| Zhang et al., 2021, China [132] | To investigate the similarities and differences between the cervical and vaginal microbiota in hrHPV-infected women in China. | 32 of the other hrHPV group (Group O) (25–45) | 20 control group (Group N) 38 HPV 16/18 group (Group H) 10 CC group (Group C) (25–45) | Cervical and vaginal swabs V3–V4 regions of the 16S rRNA gene, Illumina MiSeq platform | In the normal group and the hrHPV+ group, hrHPV16/18 infection was associated with higher microbial diversity in the healthy cervix compared to the vagina. HPV− subjects in the normal group exhibited a lower percentage of Firmicutes and a higher percentage of Proteobacteria in the normal cervix compared to the vagina. | Increase: Cancerous cervix: γ-Proteobacteria. Cancerous vagina and cervix: Prevotella. HPV16/18(+) CC and the cancerous vagina/cervix: Gardnerella and Atopobium. All hrHPV-infected vagina/cervix: Sneathia irrespective of cancerous status. Decrease: CC: Lactobacillus. Lactobacillus in cervix compared to the vagina in both hrHPV+ and hrHPV− subjects. However, this difference was not significant in the cancerous cervix. | The findings showed that the cervix and vagina had distinct compositions of the phylum Proteobacteria. Specifically, Sphingomonas, belonging to α-Proteobacteria, demonstrated potential protective effects against hrHPV infection. On the other hand Pseudomonas, in the γ-Proteobacteria group, showed a positive association with hrHPV infection and CC. |
| Kawahara et al., 2021, Japan [108] | To investigate the connections between CVM, HPV infection, and cytokine profiles in premenopausal women with CIN before and after undergoing surgical procedures such as laser cone resection, diathermy, and LEEP. | 28 Japanese patients with CIN needed surgery, 5 individuals underwent laser cone resection, 23 patients underwent LEEP with diathermy. (24–48) | 13 Japanese patients with CIN observation only (24–48) | Cervical swabs V3–V4 regions of the 16S rRNA gene, Illumina MiSeq platform | L. crispatus negatively correlated with anaerobic bacteria like Dialister, A. vaginae, Adlercreutzia, Parimonas, and Clostridium in both collections. Anaerobic bacteria (Prevotella, Dialister, A. vaginae, Sneathia, Adlercreutzia, Peptoniphilus, Megashpaera, Parvimonas, and Clostridium) positively correlated with each other and were unchanged after surgery. L. crispatus strongly associated with L. jensenii during the first collection and after surgery. | Increase: After surgery: Tenericutes, Ureaplasma Decrease: After surgery: Proteobacteria, A. vaginae, and Methylobacteriaceae | Atopobium and Gardnerella were associated with HPV and CIN. Reduced HPV infections and neoplastic lesion removal may decrease microbiota diversity. L. iners and Gardnerella disrupt the cervical barrier, while L. crispatus has a protective effect. Proinflammatory cytokines increased with anaerobic bacteria presence and inversely with Lactobacillus dominance. Surgical intervention dramatically changed the CVM and local immunity. |
| Sasivimolrattana et al., 2022, Thailand [133] | To examine the bacterial, fungal, and viral communities in the cervix of Thai patients with HPV16 and high-risk HPV infections at different precancerous stages. | 43 patients HPV+ with CIN at different stages: 22 CIN-1, 7 CIN-2, and 14 CIN-3 (23–50) | 5 CIN-1 HPV− (23–50) | Cervical swabs V1–V9 region of bacterial 16S rRNA gene; fungal ITS1 and ITS2 genes, Illumina MiSeq platform | Over the period of 5–7 years, the cervical microbiome’s categorical composition exhibited both relative stability and occasional fluctuations between a small number of defined community types. Ns. differences were observed in fungal abundance among the groups. | Increase: CIN-1, CIN-2/3 HPV+: L. iners, CIN-1 HPV− NHD: Parvimonas sp., Olsenella sp. Decrease: CIN-1 HPV−: C. albicans CIN-1, CIN-2/3 HPV+: bacterial diversity, human viral diversity | Lactobacillus sp. influenced bacterial diversity, and HPV infection impacted both bacterial and human viral diversity. Certain microorganisms showed correlations with HPV infection and dysplasia severity, suggesting their potential as diagnostic tools. |
| Shi et al., 2022, China [134] | To investigate the association between the CVM at baseline and the clearance of hrHPV infection within 12 months. | 45 HPV-cleared after 12 months (24–68) | 28 HPV-uncleared after 12 months (24–68) | Cervical swabs V4–V5 regions of 16S rRNA gene, Illumina MiSeq platform | After 12 months, patients with HSIL had slightly higher clearance rates compared to those with HPV+/LSIL, with the difference approaching statistical significance. No significant differences were observed between patients who successfully cleared HPV and those who did not among both α- and β-diversity. | Increase: HPV16 or non-Lactobacillus-dominated community state type: higher microbiome diversity; HPV-cleared: Enterococcus ASV_62 (at baseline); HPV-uncleared: L. iners (at baseline) | L. iners abundance at diagnosis was negatively related to HPV clearance over 12 months, especially in non-operative treatment patients. This highlights the potential role of the microbiota in persistent hrHPV infections. |
| Liu et al., 2022, China [135] | To explore how the vaginal microbiota contributes to reducing disease risk and identify factors affecting disease susceptibility in six Chinese nationalities (Zhang, Naxi, Yi, Bai, Lisu, and Han). | 43 HPV+ (30–50) | 39 HPV− (30–50) | Cervical swabs V4–V5 regions of 16S rRNA gene, Illumina MiSeq platform | A potential association between Prevotella and cervical disease was indicated. | Increase: HPV−: Lactobacillus, C. accolens, M. cohnii, R. bromii, L. herbarum, P. flavescens HPV+: C. flavescens, C. jeikeium, C. ihumii, C. gottingense, M. mulieris, C. acnes, P. niger, S. chromogenes, B. velezensis, C. ureolyticus, A. johnsonii, A. lwoffii, P. excrementihominis, R. pickettii, S. sanguinegens | Monitoring the microbial environment in the vagina and cervix can help identify early HPV infections and other health issues. Additionally, adjusting the microbial environment offers a potential approach to promoting vaginal and cervical health. |
| Kaelin et al., 2022, USA [109] | To investigate the relationship between the cervicovaginal DNA virome and other features of the local microenvironment, including CVM and genital inflammation, and examine these factors, which influence HPV persistence and progression to CC. | 18 HPV+ premenopausal, nonpregnant women (23–50) | 5 HPV− premenopausal, nonpregnant women (23–50) | Vaginal swabs and cervicovaginal lavage V4–V5 regions of 16S rRNA gene, Illumina MiSeq platform | HPV+ groups and certain HPV infections had more diverse microbiota compared to HPV− groups. The age group over 60 also showed higher microbiota diversity. The study highlighted the significant impact of microbiota, particularly pathogenic microorganisms, on metabolic function. | Increase: HPV+: Alphapapillomavirus | Anelloviruses were linked to genital inflammation. An association between trans-kingdom interactions, the type of microbiome profile (Lactobacillus dominated vs. non-Lactobacillus dominated), and genital inflammation. Cervicovaginal virome might play a role in microbiome changes and inflammation, potentially leading to persistent HPV infections and the development of CC. |
| Hu et al., 2022, China, Australia [136] | To investigate the association between HPV infection and CM changes, especially in relation to different HPV groups and genotypes, and the impact of the microbiota on cellular and metabolic functions; to explore microbiota changes across different age groups within a population cohort in Sanmenxia, Henan Province. | 94 HPV+ | 182 HPV− | Fluid sample after Pap Smear preparation V3–V4 regions of 16S rRNA gene, Illumina HiSeq platform | Predominant microbiota compositions included specific species: L. iners, E. coli, E. faecalis, and A. vaginae. Significant differences in microbiota diversity observed between the HPV+ group and those infected with unique-268 and multi-268 HPV strains compared to the HPV− group. Furthermore, the study revealed that women older than 60 years exhibited higher microbiota diversity compared to younger women. | Increase: HPV+: higher diversity with Bifidobacteriales, Lactobacillus, Bifidobacteriaceae, Gardnerella, Coriobacteria, A. vaginae, Clostridia, and Sneathia. Unique-268 HPV+: Betaproteobacteriales, Burkholderiaceae, Weeksellaceae, Flavobacteriales, Gardnerella, P. aeruginosa, and Mycoplasma compared to multi-268 HPV+. Multi-268 HPV+: Presence of Saccharimonadales, Saccharimonadia, Patescibacteria, Bifidobacteriales, and Bifidobacteriaceae. | Increased microbial diversity and a higher proportion of pathogenic microorganisms are likely associated with abnormalities in metabolic functions. The clinical implications of the above microbiota results under different HPV infection statuses involve the identification of potential biomarkers for diagnosing cases. |
| Molina et al., 2022, The Netherlands [31] | To characterize CSTs in samples from hrHPV− women and hrHPV+ women with and without cervical lesions, using ciRNAseq for high-resolution CVM profiling. | 200 HPV+ samples, divided into 100 LSIL and 100 HSIL (CIN-2+) cases; 44 HPV− | 297 women without cervical lesions | Cervical smears CiRNAseq, Illumina Nextseq500 platform | Cervicovaginal microbes were categorized into five distinct CSTs, characterized by their microbial community composition and abundance. CSTs I, III, and IV based on intra-CST differences with respect to abundances of L. acidophilus (CSTs I-A vs. I-B and CSTs III-A vs. III-B), L. iners (CSTs I-A vs. I-B and CSTs III-A vs. III-B), and M. genomosp type 1 (CSTs IV-A vs. IV-B). CST V was associated with uninfected conditions, and CST IV-A was associated with hrHPV-induced cervical disease. | Increase: HPV−: L. acidophilus hrHPV+: CST IV in NILM, LSIL and HSIL groups Decrease: hrHPV+: L. crispatus (CST I) in NILM, LSIL, and HSIL groups; CST V in HSIL | An agreement on CST designation based on high-resolution CVM profiling is promoted, considering microbial dominance, composition, abundance, and diversity. Microbial dynamics occurring in the CVM are suggested by this classification. The data emphasize the identification of commonly overlooked bacterial species, such as L. acidophilus and M. genomosp type 1, which are relevant for cervical health and microbial relationships. High-resolution microbiome profiling for appropriate classification is necessary. |
| Fang et al., 2022, China [107] | To examine the composition and function of the CM and its association with hrHPV infection and find ways to prevent persistent hrHPV infection by restoring a healthy microbial balance in the reproductive tract. | 20 hrHPV+ (25–45) | 20 hrHPV− (25–45) | Cervical swabs V3–V4 regions of 16S rRNA gene, Illumina Novaseq 6000 platform | The study highlighted significant differences in the cervical microbiome between hrHPV-infected and uninfected women. Notably, three species, L. crispatus, L. jensenii, and L. helveticus, stood out as potential microbial targets for future treatment due to their biomarker significance. | Increase: hrHPV+: Gardnerella, Atopobium, and Bifidobacterium hrHPV−: L. crispatus, L. jensenii, L. helveticus Decrease: hrHPV+: Lactobacillus, L. crispatus hrHPV−: Gardnerella, Atopobium | By utilizing both 16S rRNA gene and metagenomic sequencing, a comprehensive understanding of the diversity, composition, and function of CM was achieved. |
| Li et al., 2022, China [110] | To examine the CVM before and after treatments and explore its association with HPV persistence. | 26 HPV16+ and CIN-1, 34 HPV16+ and CIN-2/3, 6 HPV16+ and squamous cell carcinoma. (<29 and >60) | 25 healthy controls (<29 and >60) | Cervical swabs V3–V4 regions of 16S rRNA gene, MiSeq Illumina platform | Firmicutes, Bacteroidetes Proteobacteria, Actinobacteria, and Fusobacteria were dominant. Following clinical treatment, there was a tendency towards increased abundance of Lactobacillus and decreased abundance of non-Lactobacillus genera. | Increase: The dominant bacteria in CST2 and CST4, such as Burkholderia, G. vaginalis, Pseudomonas, E. coli, Atopobium, S. amnii, and Prevotella, were associated with bacterial vaginosis and could potentially contribute to the development of CIN. Decrease: Non-Lactobacillus genera, including Burkholderia and Pseudomonas | The study revealed that advanced CIN lesions are associated with increased CVM diversity. After treatment, a reduced diversity in the CVM was observed in CINs. This suggests that both antiviral and local excisional treatments effectively clear HPV16 infection and aid in the recovery of the CVM. |
| Guo et al., 2022, China [137] | To examine the differences in CVM among HPV−, HPV+NoSIL, HPV+LSIL, and HPV+HSIL groups; to interpret the association of CVM with HPV infection and SIL level. | 40 HPV+NoSIL 28 HPV+LSIL 51 HPV+HSIL (19–50) | 30 HPV− (19–50) | Cervical brushes V3–V5 region of 16S rRNA gene, NovaSeq Illumina platform | The analysis at the phylum level revealed higher diversity of taxonomic phylum in the HPV+HSIL group compared to the other three groups. This included increased levels of Fusobacteria, Proteobacteria, and Tenericutes. | Increase: Women with SIL: non-Lactobacillus CVM compared to women in the HPV− and HPV+NoSIL groups. HPV+HSIL group: Megasphaera. Decrease: HPV+HSIL group: Enterococcus. | There were observed significant differences in the CVM among women with HSIL, supporting the association between the CVM and clinical outcomes of HPV infection. Possibly, the CVM may influence the risk of persistence of pre-existing HPV and SIL progression, rather than the risk of HPV acquisition. |
| Vikramdeo et al., 2022, USA [138] | To analyze cervical intraepithelial lesions from women with different ethnic backgrounds in the United States, i.e., Hispanic/Latina (HIS), African American (AA), and Caucasian American (CA) and their resident microbial compositions, as these groups show variations in CC incidence and outcomes. | 36 CIN tissues from various grades (CIN-1-CIN-3). 12 CA, 12 AA, 12 HIS (21–62) | Biopsy specimens V4 region of 16S rRNA gene, MiSeq Illumina platform | Exclusively in women with a histopathological diagnosis of CIN, a unique niche of 27 microbes was identified. A group of 8 microbiota (Rubellimicrobium, Podobacter, Brevibacterium, Paracoccus, Atopobium, Brevundimonous, Comamonous, and Novospingobium) was exclusively detected in the CIN lesions obtained from AA and CA women. | Increase: Micrococcus in AA and HIS compared to CA. Prevotella in HIS compared to CA and AA. Rubellimicrobium, Podobacter, Brevibacterium, Paracoccus, Atopobium, Brevundimonous, Comamonous, and Novospingobium were exclusively detected only in CIN samples of AA and CA. Decrease: Lactobacillus in AA and HIS compared to CA | The study identified distinct microbiota abundance in women from different racial groups with cervical preneoplasia. These differences may play a role in diverse CC risk outcomes and disease progression. | |
| Wang et al., 2022, China [139] | To study the CM composition, diversity, and signaling pathways in patients with CIN and CC. | 9 CIN-1, 11 CIN-2, 17 CIN-3, and 9 CC samples (22–62) | 14 normal samples (22–62) | Biopsy specimens V4 region of 16S rRNA gene, MiSeq Illumina platform | Β-, γ-, and α-Proteobacteria, Bacillus, and Clostridium were the dominant strains in the normal group, CIN, group and CC group. Lactobacillus was the dominant strain in each group, although some samples in the normal group did not exhibit dominant Lactobacillus. A predictive model was established to assess the potential for malignant transformation from the perspective of cervical microbial genes. | Increase: The normal group: mainly composed of Gammaproteobacteria. CIN-1 and CIN-2 groups: dominated by Sphingomyces. CIN-3 and CC groups: predominantly composed of Bacteroides. | The close relationship between vaginal microecology and CIN was established. This study identified key genes from the cervical microbial community associated with CIN’s occurrence. An early warning model was established, which includes the ABCG2+PCNA+TDG genes and offers a target for clinical prediction and intervention to prevent the malignant transformation of CIN through cervical microbiological-related genes. |
| Liu et al., 2022, China [140] | To explain the connections between various bacterial species and the expression of HPV oncogenes at distinct stages of CC. | 40 hrHPV and CIN (50.00±9,95) 41 CC (54.20±7.79) | 34 hrHPV without CIN (49.74±11.49) | Cervical brushes Shotgun metagenomics, Illumina HiSeq 2500 platform | Positive correlation between the presence of HPV oncogene expression and specific bacterial species, particularly within the Sneathia and Peptostreptococcus genera. Significant increase in aerobic and anaerobic bacteria, as well as a notable rise in both prevalence and expression of HPV E6/E7 oncogenes. Clear decline in the abundance of Lactobacillus genus and species, along with the severity of cervical lesions. | Increase: HPV+: Lactobacillus genus (65.96% in HPV, 27.81% in CIN, and 9.19% in CC), Gardnerella genus (7.81%, 24.25%, and 11.24%), Prevotella genus (2.50%, 6.92%, 11.69%) in comparing to CIN and CC Decrease: HPV (+): L. iners (33.57%, 18.59%, and 7.47%, respectively) and L. crispatus (25.73%, 6.99%, and 0.82%, respectively), G. vaginalis (7.72%, 23.85%, 11.11%, respectively), P. bivia (0.54%, 3.09%, 6.86%) comparing to CIN and CC | A notable decrease in the abundance of Lactobacillus genus and species, coupled with an increase in both anaerobic and aerobic bacteria with an elevation of HPV E6/E7 and the expression of oncogenes observed along with the severity of lesions of the cervix. The overexpression of HPV oncogenes showed associations with specific bacterial species at different stages of CC. |
| Stoian et al., 2023, Romania [35] | To characterize the CVM in cervical lesion progression and HPV infection status. | 76 HPV+ including: 17 ASCUS, 13 ASCH, 18 LSIL, 10 HSIL, 9 SCC, 9 NILM | 11 HPV− | Cervical swabs V3–V4 regions of 16S rRNA gene, MiSeq Illumina platform | Unique pattern in a specific group regarding Lactobacillus species, different from other populations. Presence of L. iners with the absence of L. crispatus, along with Atopobium spp., Prevotella spp., and Gardnerella spp., could be indicative of severe cervical lesions. Strong link between microbiota diversity, HPV infection, and the progression of cervical lesions. | Increase: HPV+: NILM: L. iners; ASCUS: Lactobacillus unclassified; HSIL: Gardnerella; SCC: Prevotella; ASCH and SCC: E. coli cft073; LSIL: E. faecalis. HPV−: higher frequency of the Lactobacillales Decrease: HPV+: SCC: Lactobacillus | HSIL and SCC exhibited higher microbiota diversity in comparison to those with NILM results. Absence of L. crispatus and the presence of L. iners in HPV− individuals with normal Pap results. Among HSIL patients, a few cases demonstrated the presence of Sneathia spp. with relatively low numbers, while Gardnerella spp. and E. coli were the predominant components of their microbiota. |
| Vargas-Robles et al., 2023, Puerto Rico [141] | To assess differences in the CVM in Puerto Rican women, pregnant, nonpregnant, or menopausal, with or without HPV infections. | 133 from total of 294 hrHPV+, including 84 nonpregnant 21 pregnant 28 menopause | 74 from 294 HPV− | Cervical swabs V4 region of 16S rRNA gene, MiSeq Illumina platform | CVM was dominated by L. iners. Pregnant women in second and third trimesters showed a reduction in diversity and abundance of bacteria SCC non-Lactobacillus-dominant linked to bacterial vaginosis. Postmenopausal women displayed higher alpha diversity and a greater proportion of facultative and strictly anaerobic bacteria. Greater alpha diversity was associated with cervical lesions, but no significant associations were found between the microbiota and HPV infection, regardless of whether it was high-risk or low-risk HPV types. | Increase: hrHPV+: Clostridium spp. hrHPV+ LSIL: E. coli Decrease: hrHPV+: Ureaplasma | Women in Puerto Rico typically had a CVM dominated by L. iners or a diverse microbial profile, irrespective of their life stage. A high prevalence of hrHPV and less stable bacterial profiles might contribute to the increased risk of CC observed in the population. |
| Teka et al., 2023, Ethiopia [142] | To analyze and describe the CVM in women with premalignant dysplasia or invasive CC in comparison to healthy women. | 93 HPV+, including 60 CC patients without any treatment | 27 HPV− | Cervical brushes/swabs V4 region of 16S rRNA gene, MiSeq Illumina platform | Patients with CC exhibited higher alpha diversity compared to individuals with dysplasia and healthy women. Significant differences in beta diversity when comparing CC patients with the other groups. The microbiota composition varied between the dysplasia and CC groups. | Increase: HPV+: Porphyromonas, Peptoniphilus HPV+ CC: L. iners Dysplasia and HPV−: Lactobacillus CC: Porphyromonas, Prevotella, Bacteroides, Anaerococcus | The diversity and composition of the CVM increased from dysplasia to cancer. Women with dysplasia had higher levels of L. iners compared to healthy women. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowienka-Stodolak, M.; Bagińska-Drabiuk, K.; Szubert, S.; Hennig, E.E.; Horala, A.; Dąbrowska, M.; Micek, M.; Ciebiera, M.; Zeber-Lubecka, N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers 2024, 16, 399. https://doi.org/10.3390/cancers16020399
Głowienka-Stodolak M, Bagińska-Drabiuk K, Szubert S, Hennig EE, Horala A, Dąbrowska M, Micek M, Ciebiera M, Zeber-Lubecka N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers. 2024; 16(2):399. https://doi.org/10.3390/cancers16020399
Chicago/Turabian StyleGłowienka-Stodolak, Maria, Katarzyna Bagińska-Drabiuk, Sebastian Szubert, Ewa E. Hennig, Agnieszka Horala, Michalina Dąbrowska, Martyna Micek, Michał Ciebiera, and Natalia Zeber-Lubecka. 2024. "Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies" Cancers 16, no. 2: 399. https://doi.org/10.3390/cancers16020399
APA StyleGłowienka-Stodolak, M., Bagińska-Drabiuk, K., Szubert, S., Hennig, E. E., Horala, A., Dąbrowska, M., Micek, M., Ciebiera, M., & Zeber-Lubecka, N. (2024). Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers, 16(2), 399. https://doi.org/10.3390/cancers16020399