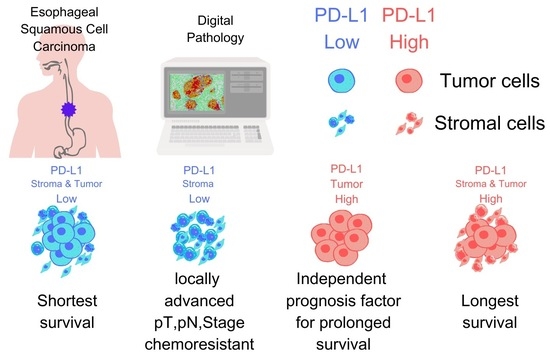
Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry
2.3. Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. PD-L1 Expression in Stroma, Tumor, and Stroma + Tumor
3.2. Survival Analysis Based on PD-L1 H-Score
3.3. Multivariate Analysis of Clinicopathological Factors
3.4. Correlation Analysis of PD-L1 Expression and Clinicopathological Factors
3.5. PD-L1 Expression and Its Association with Recurrence Patterns and Metastatic Sites
3.6. Comparison of Survival Times Based on PD-L1 Expression Patterns in Stroma and Tumor
3.7. PD-L1 Expression and Post-Recurrence Treatment Efficacy: CTx and Immune Checkpoint Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Takahashi, A.; Ueno, H.; Kakeji, Y.; Hasegawa, H.; Eguchi, S.; Goi, T.; Saiura, A.; Sasaki, A.; Takiguchi, S.; et al. Annual report on National Clinical Database 2020 for gastroenterological surgery in Japan. Ann. Gastroenterol. Surg. 2023, 7, 367–406. [Google Scholar] [CrossRef]
- Booka, E.; Takeuchi, H.; Morita, Y.; Hiramatsu, Y.; Kikuchi, H. What is the best reconstruction procedure after esophagectomy? A meta-analysis comparing posterior mediastinal and retrosternal approaches. Ann. Gastroenterol. Surg. 2023, 7, 553–564. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, S.; Zhang, L.; Guo, S.; Shen, J.; Li, Q.; Yang, H.; Feng, Y.; Liu, M.; Lin, S.H.; et al. Recurrence Risk Based on Pathologic Stage after Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma: Implications for Risk-Based Postoperative Surveillance Strategies. Ann. Surg. Oncol. 2018, 25, 3639–3646. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- PD-L1 IHC 22C3 pharmDx Interpretation Manual—Esophageal Cancer. Available online: https://www.agilent.com/cs/library/usermanuals/public/29439-d67239-pd-l1-ihc22c3-ec-kn590-int-man-en.pdf (accessed on 25 July 2023).
- McCarty, K.S., Jr.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; Budwit, D.A.; Creasman, W.T.; et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986, 46, 4244s–4248s. [Google Scholar] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Xu, J.; Zhou, Z.G.; Jin, L.L.; Yu, X.J.; Xiao, G.; Lin, J.; Zhuang, S.M.; Zhang, Y.J.; Zheng, L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br. J. Cancer 2018, 119, 80–88. [Google Scholar] [CrossRef]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Zhai, Q.; Fan, J.; Lin, Q.; Liu, X.; Li, J.; Hong, R.; Wang, S. Tumor stromal type is associated with stromal PD-L1 expression and predicts outcomes in breast cancer. PLoS ONE 2019, 14, e0223325. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.L. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 2019, 136, 136–144. [Google Scholar] [CrossRef]
- Teramoto, K.; Igarashi, T.; Kataoka, Y.; Ishida, M.; Hanaoka, J.; Sumimoto, H.; Daigo, Y. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer 2019, 137, 56–63. [Google Scholar] [CrossRef]
- Wu, X.; Ke, X.; Ni, Y.; Kuang, L.; Zhang, F.; Lin, Y.; Lin, W.; Xiong, X.; Huang, H.; Lin, X.; et al. Tumor-Infiltrating Immune Cells and PD-L1 as Prognostic Biomarkers in Primary Esophageal Small Cell Carcinoma. J. Immunol. Res. 2020, 2020, 8884683. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Long, Q.; Li, Q.; Tian, J.; Liu, T.; Wu, Y.; Ding, Z. Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J. Immunother. Cancer 2021, 9, PMC8504357. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, S.; Morita, Y.; Matsumoto, A.; Ida, S.; Muraki, R.; Kitajima, R.; Takeda, M.; Kikuchi, H.; Hiramatsu, Y.; Takeuchi, H. Tenascin C in pancreatic cancer-associated fibroblasts enhances epithelial mesenchymal transition and is associated with resistance to immune checkpoint inhibitor. Am. J. Cancer Res. 2023, 13, 5641–5655. [Google Scholar] [PubMed]
- Okadome, K.; Baba, Y.; Nomoto, D.; Yagi, T.; Kalikawe, R.; Harada, K.; Hiyoshi, Y.; Nagai, Y.; Ishimoto, T.; Iwatsuki, M.; et al. Prognostic and clinical impact of PD-L2 and PD-L1 expression in a cohort of 437 oesophageal cancers. Br. J. Cancer 2020, 122, 1535–1543. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, Y.; Tang, S.; Zhang, G.; Lin, Q.; Xu, Y.; Shang, J. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol. Lett. 2019, 17, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, F.; Shao, F.; Wang, P.; Li, Z.; Yang, X.; He, Z.; Shi, S.; Gao, Y.; He, J. PD-L1 expression on tumor cells associated with favorable prognosis in surgically resected esophageal squamous cell carcinoma. Hum. Pathol. 2019, 84, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe, H.; Hashimura, M.; Matsumoto, T.; Yokoi, A.; Nakagawa, M.; Ishibashi, Y.; Ito, T.; Ohhigata, K.; Saegusa, M. A combination of stromal PD-L1 and tumoral nuclear β-catenin expression as an indicator of colorectal carcinoma progression and resistance to chemoradiotherapy in locally advanced rectal carcinoma. J. Pathol. Clin. Res. 2022, 8, 458–469. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Z.; Gu, J.; Li, Z.; Xu, X.; Xue, C.; Li, X.; Zhao, L.; Zhou, J.; Bai, C.; et al. Cancer-Associated Fibroblasts Promote the Upregulation of PD-L1 Expression Through Akt Phosphorylation in Colorectal Cancer. Front. Oncol. 2021, 11, 748465. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ishida, M.; Yanai, H.; Tsuta, K.; Sekimoto, M.; Sugie, T. Prognostic significance of PD-L1-positive cancer-associated fibroblasts in patients with triple-negative breast cancer. BMC Cancer 2021, 21, 239. [Google Scholar] [CrossRef]
- Farlow, J.L.; Brenner, J.C.; Lei, Y.L.; Chinn, S.B. Immune deserts in head and neck squamous cell carcinoma: A review of challenges and opportunities for modulating the tumor immune microenvironment. Oral. Oncol. 2021, 120, 105420. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]


| Variables | N | (%) |
|---|---|---|
| Age | ||
| under 70 y.o | 114 | (59%) |
| over and 70 y.o | 80 | (41%) |
| Sex | ||
| male | 169 | (88%) |
| female | 25 | (13%) |
| Tumor Location | ||
| Ce | 4 | (2%) |
| Ut | 22 | (11%) |
| Mt | 95 | (49%) |
| Lt | 72 | (37%) |
| Ae | 1 | (1%) |
| Differentiation | ||
| differentiated | 140 | (73%) |
| include poorly differentiated | 41 | (21%) |
| NA | 13 | (7%) |
| Preoperative treatment | ||
| None | 97 | (50%) |
| Chemotherapy | 87 | (45%) |
| Chemotherapy+Radiation | 10 | (5%) |
| pT | ||
| T1 | 94 | (49%) |
| T2 | 26 | (13%) |
| T3 | 74 | (38%) |
| pN | ||
| N0 | 71 | (37%) |
| N1 | 65 | (34%) |
| N2 | 34 | (18%) |
| N3 | 24 | (12%) |
| Lymphatic invasion | ||
| negative | 70 | (36%) |
| positive | 124 | (64%) |
| Venous invasion | ||
| negative | 55 | (28%) |
| positive | 139 | (72%) |
| Intramural metastasis | ||
| negative | 176 | (91%) |
| positive | 18 | (9%) |
| pStage * | ||
| I | 55 | (28%) |
| II | 43 | (22%) |
| III | 59 | (31%) |
| IVA | 37 | (19%) |
| Total recurrence cases | 85 | (44%) |
| Lymphogenous metastasis | ||
| negative | 16 | (19%) |
| positive | 69 | (81%) |
| Hematogenous metastasis | ||
| negative | 33 | (39%) |
| positive | 52 | (61%) |
| Locoregional recurrence | ||
| negative | 76 | (89%) |
| positive | 9 | (11%) |
| Pleural/peritoneal dissemination | ||
| negative | 66 | (78%) |
| positive | 19 | (22%) |
| Variables | N | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | ||||
| Age | |||||||||
| under 70 y.o | 114 | 1.00 | 0.48 | 1.30 | 0.355 | ||||
| over and 70 y.o | 80 | 0.79 | |||||||
| Sex | |||||||||
| male | 169 | 1.00 | 0.31 | 1.47 | 0.321 | ||||
| female | 25 | 0.67 | |||||||
| Differentiation | |||||||||
| differentiated | 140 | 1.00 | 0.95 | 2.79 | 0.077 | ||||
| include poorly diff. | 41 | 1.63 | |||||||
| Preoperative treatment | |||||||||
| none | 97 | 1.00 | 1.77 | 4.97 | <0.001 | 1.00 | 0.95 | 3.08 | 0.075 |
| chemotherapy(+/-Radiation) | 97 | 2.97 | 1.71 | ||||||
| pT * | |||||||||
| <T2 | 94 | 1.00 | 2.54 | 7.82 | <0.001 | 1.00 | 0.95 | 3.65 | 0.069 |
| T2 and over | 100 | 4.46 | 1.86 | ||||||
| pN * | |||||||||
| negative | 71 | 1.00 | 2.80 | 12.28 | <0.001 | 1.00 | 1.12 | 6.52 | 0.027 |
| positive | 123 | 5.86 | 2.70 | ||||||
| Lymphatic invasion | |||||||||
| negative | 70 | 1.00 | 1.77 | 6.11 | <0.001 | 1.00 | 0.47 | 2.27 | 0.943 |
| positive | 124 | 3.28 | 1.03 | ||||||
| Venous invasion | |||||||||
| negative | 55 | 1.00 | 1.96 | 9.35 | <0.001 | 1.00 | 0.61 | 3.89 | 0.357 |
| positive | 139 | 4.23 | 1.55 | ||||||
| Intramural metastasis | |||||||||
| negative | 176 | 1.00 | 1.58 | 5.28 | <0.001 | 1.00 | 0.80 | 2.93 | 0.201 |
| positive | 18 | 2.88 | 1.53 | ||||||
| PD-L1 Stroma | |||||||||
| low | 117 | 1.00 | 0.17 | 0.55 | <0.001 | 1.00 | 0.21 | 1.17 | 0.108 |
| high | 77 | 0.30 | 0.49 | ||||||
| PD-L1 Tumor | |||||||||
| low | 102 | 1.00 | 0.30 | 0.82 | 0.006 | 1.00 | 0.27 | 0.83 | 0.010 |
| high | 92 | 0.49 | 0.47 | ||||||
| PD-L1 Stroma+Tumor | |||||||||
| low | 143 | 1.00 | 0.20 | 0.80 | 0.010 | 1.00 | 0.45 | 3.84 | 0.616 |
| high | 51 | 0.40 | 1.32 | ||||||
| Variables | N | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | ||||
| Age | |||||||||
| under 70 y.o | 114 | 1.00 | 0.52 | 1.26 | 0.344 | ||||
| over and 70 y.o | 80 | 0.81 | |||||||
| Sex | |||||||||
| male | 169 | 1.00 | 0.25 | 1.18 | 0.121 | ||||
| female | 25 | 0.54 | |||||||
| Differentiation | |||||||||
| differentiated | 140 | 1.00 | 1.05 | 2.74 | 0.031 | 1.00 | 0.71 | 1.92 | 0.534 |
| include poorly diff. | 41 | 1.70 | 1.17 | ||||||
| Preoperative treatment | |||||||||
| none | 97 | 1.00 | 1.91 | 4.79 | <0.001 | 1.00 | 0.99 | 2.98 | 0.051 |
| chemotherapy(+/−Radiation) | 97 | 3.03 | 1.72 | ||||||
| pT * | |||||||||
| <T2 | 94 | 1.00 | 2.36 | 6.13 | <0.001 | 1.00 | 0.89 | 2.91 | 0.114 |
| T2 and over | 100 | 3.80 | 1.61 | ||||||
| pN * | |||||||||
| negative | 71 | 1.00 | 2.68 | 9.15 | <0.001 | 1.00 | 1.17 | 5.58 | 0.018 |
| positive | 123 | 4.96 | 2.56 | ||||||
| Lymphatic invasion | |||||||||
| negative | 70 | 1.00 | 1.66 | 4.82 | <0.001 | 1.00 | 0.53 | 2.15 | 0.850 |
| positive | 124 | 2.83 | 1.07 | ||||||
| Venous invasion | |||||||||
| negative | 55 | 1.00 | 1.71 | 5.80 | <0.001 | 1.00 | 0.62 | 3.03 | 0.436 |
| positive | 139 | 3.14 | 1.37 | ||||||
| Intramural metastasis | |||||||||
| negative | 176 | 1.00 | 1.44 | 4.56 | 0.001 | 1.00 | 0.70 | 2.44 | 0.397 |
| positive | 18 | 2.56 | 1.31 | ||||||
| PD-L1 Stroma | |||||||||
| low | 117 | 1.00 | 0.24 | 0.65 | <0.001 | 1.00 | 0.43 | 1.48 | 0.465 |
| high | 77 | 0.39 | 0.79 | ||||||
| PD-L1 Tumor | |||||||||
| low | 108 | 1.00 | 0.35 | 0.87 | 0.010 | 1.00 | 0.32 | 0.91 | 0.022 |
| high | 87 | 0.55 | 0.54 | ||||||
| PD-L1 Stroma+Tumor | |||||||||
| low | 163 | 1.00 | 0.15 | 0.80 | 0.013 | 1.00 | 0.33 | 2.77 | 0.930 |
| high | 31 | 0.35 | 0.95 | ||||||
| Variables | PD-L1 Stroma | PD-L1 Tumor | PD-L1 Stroma + Tumor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low N = 117 | High N = 77 | p-Value | Low N = 102 | High N = 92 | p-Value | Low N = 143 | High N = 51 | p-Value | |
| Age | |||||||||
| under 70 y.o | 37% | 22% | 0.333 | 31% | 27% | 0.756 | 44% | 14% | 0.514 |
| over and 70 y.o | 23% | 18% | 21% | 20% | 29% | 12% | |||
| Sex | |||||||||
| male | 55% | 32% | 0.074 | 47% | 40% | 0.357 | 65% | 22% | 0.237 |
| female | 6% | 7% | 6% | 7% | 8% | 5% | |||
| Tumor Location | |||||||||
| Ce | 2% | 1% | 0.736 | 2% | 1% | 0.298 | 2% | 1% | 0.498 |
| Ut | 7% | 4% | 7% | 5% | 8% | 4% | |||
| Mt | 29% | 20% | 28% | 21% | 37% | 12% | |||
| Lt | 22% | 15% | 16% | 21% | 27% | 10% | |||
| Ae | 0% | 1% | 0% | 1% | 0% | 1% | |||
| Differentiation | |||||||||
| differentiated | 42% | 30% | 0.701 | 36% | 37% | 0.267 | 53% | 19% | 0.962 |
| include poorly differentiated | 14% | 7% | 12% | 9% | 15% | 6% | |||
| NA | 4% | 3% | 5% | 2% | 5% | 2% | |||
| Preoperative treatment | |||||||||
| None | 28% | 22% | 0.394 | 28% | 22% | 0.538 | 35% | 15% | 0.510 |
| Chemotherapy | 29% | 16% | 22% | 23% | 35% | 10% | |||
| Chemotherapy+Radiation | 4% | 2% | 3% | 2% | 4% | 1% | |||
| pT * | |||||||||
| T1 | 22% | 27% | <0.001 | 25% | 23% | 0.988 | 32% | 16% | 0.051 |
| T2 | 10% | 4% | 7% | 6% | 10% | 3% | |||
| T3 | 29% | 9% | 20% | 18% | 31% | 7% | |||
| pN * | |||||||||
| N0 | 15% | 21% | <0.001 | 18% | 19% | 0.362 | 23% | 14% | 0.041 |
| N1 | 22% | 11% | 21% | 13% | 26% | 7% | |||
| N2 | 13% | 5% | 8% | 9% | 14% | 3% | |||
| N3 | 10% | 3% | 6% | 6% | 10% | 2% | |||
| Lymphatic invasion | |||||||||
| negative | 16% | 20% | <0.001 | 19% | 18% | 0.810 | 24% | 12% | 0.057 |
| positive | 44% | 20% | 34% | 30% | 50% | 14% | |||
| Venous invasion | |||||||||
| negative | 10% | 19% | <0.001 | 13% | 15% | 0.352 | 16% | 12% | 0.002 |
| positive | 51% | 21% | 39% | 32% | 57% | 14% | |||
| Intramural metastasis | |||||||||
| negative | 54% | 37% | 0.278 | 47% | 43% | 0.790 | 67% | 24% | 0.880 |
| positive | 7% | 3% | 5% | 4% | 7% | 3% | |||
| pStage * | |||||||||
| I | 8% | 20% | <0.001 | 14% | 14% | 0.658 | 16% | 12% | 0.013 |
| II | 17% | 5% | 11% | 11% | 17% | 5% | |||
| III | 20% | 10% | 18% | 12% | 24% | 7% | |||
| IVA | 15% | 4% | 10% | 9% | 16% | 3% | |||
| PD-L1 Stroma | PD-L1 Tumor | PD-L1 Stroma + Tumor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Low N = 65 | High N = 20 | p-Value | Low N = 52 | High N = 33 | p-Value | Low N = 79 | High N = 6 | p-Value |
| Lymphogenous metastasis | |||||||||
| negative | 22% | 10% | 0.248 | 23% | 12% | 0.339 | 20% | 0% | 0.135 |
| positive | 78% | 90% | 77% | 88% | 80% | 100% | |||
| Hematogenous metastasis | |||||||||
| negative | 35% | 50% | 0.241 | 40% | 36% | 0.247 | 38% | 50% | 0.127 |
| positive | 65% | 50% | 60% | 64% | 62% | 50% | |||
| Locoregional recurrence | |||||||||
| negative | 86% | 100% | 0.078 | 88% | 91% | 0.386 | 89% | 100% | 0.289 |
| positive | 14% | 0% | 12% | 9% | 11% | 0% | |||
| Pleural/peritoneal dissemination | |||||||||
| negative | 78% | 75% | 0.745 | 79% | 76% | 0.739 | 78% | 67% | 0.503 |
| positive | 22% | 25% | 21% | 24% | 22% | 33% | |||
| Total recurrence types | |||||||||
| 1 | 45% | 55% | 0.515 | 46% | 48% | 0.139 | 43% | 50% | 0.742 |
| 2 | 37% | 25% | 38% | 27% | 32% | 17% | |||
| 3 | 14% | 20% | 15% | 15% | 13% | 33% | |||
| 4 | 5% | 0% | 0% | 9% | 3% | 0% | |||
| Total metastatic sites | |||||||||
| 1 | 40% | 55% | 0.352 | 44% | 42% | 0.318 | 41% | 50% | 0.115 |
| 2 | 22% | 10% | 25% | 9% | 19% | 0% | |||
| 3 | 23% | 20% | 19% | 27% | 19% | 17% | |||
| 4 | 9% | 5% | 8% | 9% | 8% | 17% | |||
| 5 | 6% | 5% | 4% | 9% | 4% | 17% | |||
| 6 | 0% | 5% | 0% | 3% | 0% | 0% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Booka, E.; Furuhashi, S.; Sakai, Y.; Sekimori, K.; Haneda, R.; Fujihiro, M.; Matsumoto, T.; Morita, Y.; Kikuchi, H.; et al. Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers 2024, 16, 1135. https://doi.org/10.3390/cancers16061135
Murakami T, Booka E, Furuhashi S, Sakai Y, Sekimori K, Haneda R, Fujihiro M, Matsumoto T, Morita Y, Kikuchi H, et al. Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers. 2024; 16(6):1135. https://doi.org/10.3390/cancers16061135
Chicago/Turabian StyleMurakami, Tomohiro, Eisuke Booka, Satoru Furuhashi, Yuki Sakai, Kenichi Sekimori, Ryoma Haneda, Mayu Fujihiro, Tomohiro Matsumoto, Yoshifumi Morita, Hirotoshi Kikuchi, and et al. 2024. "Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma" Cancers 16, no. 6: 1135. https://doi.org/10.3390/cancers16061135
APA StyleMurakami, T., Booka, E., Furuhashi, S., Sakai, Y., Sekimori, K., Haneda, R., Fujihiro, M., Matsumoto, T., Morita, Y., Kikuchi, H., Hiramatsu, Y., Baba, S., & Takeuchi, H. (2024). Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers, 16(6), 1135. https://doi.org/10.3390/cancers16061135