Ovarian Causes of Pseudomyxoma Peritonei (PMP)—A Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
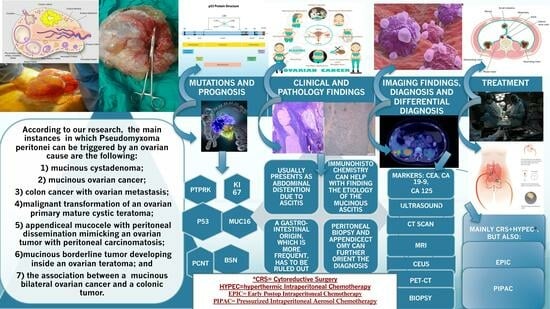
3.1. Ovarian Causes of PMP
3.1.1. Mucinous Cystadenoma
3.1.2. Mucinous Ovarian Cancer
3.1.3. Colon Cancer with Ovarian Metastasis
3.1.4. Malignant Transformation of an Ovarian Primary Mature Cystic Teratoma (MCT)
3.1.5. Appendiceal Mucocele with Peritoneal Dissemination Mimicking an Ovarian Tumor with Peritoneal Carcinomatosis
3.1.6. A Mucinous Borderline Tumor Developing inside an Ovarian Teratoma
3.1.7. The Association between a Mucinous Bilateral Ovarian Cancer and a Colonic Tumor
3.2. General Features of PMP with an Ovarian Origin
3.2.1. Mutations and Prognostics
3.2.2. Clinical and Pathology Findings
3.2.3. Imaging Findings, Diagnosis, and Differential Diagnosis
3.2.4. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Taher, R.; Gray, D.; Ramage, J. The Quality of Life of Pseudomyxoma Peritonei Patients: A Scoping Review. Eur. J. Cancer Care 2024, 2024, 8137209. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Tsapakidis, K.; Tolia, M.; Kiakou, M.; Galanopoulos, M.; Aravantinou-Fatorou, E.; Baxevanos, P.; Papadopoulos, V.; Tountziaris, C.; Nikolaou, M.; et al. Pseudomyxoma Peritonei: A Challenging Clinical Diagnosis. Case Report and Review of the Literature. Cancer Diagn. Progn. 2024, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Villarejo-Campos, P.; García-Arranz, M.; Qian, S.; de los Galanes, S.J.; Domínguez-Prieto, V.; Vélez-Pinto, J.F.; Castellano, I.G.; Guadalajara, H.; García-Olmo, D. Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei. J. Clin. Med. 2023, 12, 4007. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Shi, F.; Li, X.; Yu, C.; Lin, Y.; Li, Y.; Jin, M. Clinicopathological Characteristics of Pseudomyxoma Peritonei Originated from Ovaries. Cancer Manag. Res. 2020, 12, 7569–7578. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Roy, D.S.; Maroo, V.; Mandal, M. Incidental pathologies in appendectomy specimens—An interesting series of cases in a tertiary care center in Eastern India. Asian J. Med. Sci. 2023, 14, 278. [Google Scholar] [CrossRef]
- Martin, S.; Fica, S.; Parfeni, O.; Popa, L.; Manuc, T.; Rizea, O.; Lupescu, I.; Gherghe, M.; Becheanu, G.; Croitoru, A. Somatostatinoma and Neurofibromatosis Type 1-A Case Report and Review of the Literature. Diagnostics 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Purwoto, G.; Anggraeni, T.D.; Rustamadji, P.; Surya, I.U.; Julianti, K.; Herlambang, N. Histopathological discrepancy and variation of surgical management in mucinous ovarian cystadenoma and pseudomyxoma peritonei. Int. J. Surg. Case Rep. 2022, 94, 107141. [Google Scholar] [CrossRef] [PubMed]
- Tjokroprawiro, B.A.; Nugroho, H.; Indraprasta, B.R. Huge and Unique Pseudomyxoma Peritonei. J. Obstet. Gynecol. India 2022, 72, 268–269. [Google Scholar] [CrossRef]
- Yeom, J.; Lee, S.; Kim, Y. Hypercalcemia associated with primary mucinous ovarian tumor followed by pseudomyxoma peritonei can be fatal: A case report. Eur. J. Gynaecol. Oncol. 2020, 5, 2020. [Google Scholar] [CrossRef]
- Sarkar, A.; Mahapatra, M.; Parija, J. A Case of Mucinous Neoplasm of Appendix with Pseudomyxoma Peritonei and Ovarian Metastasis. J. South Asian Fed. Obstet. Gynaecol. 2023, 15, 605–606. [Google Scholar] [CrossRef]
- Yehya, M.; Denson, M.; Moszczynski, Z. Multi-origin mucinous neoplasm: Should we prophylactically remove the appendix in the setting of mucinous ovarian tumors? Int. J. Surg. Case Rep. 2020, 66, 326–329. [Google Scholar] [CrossRef]
- Matsuzono, T.; Hon, L.W.; May, C.Y.M. Is appendectomy a must for borderline and invasive mucinous ovarian tumour? Eur. J. Gynaecol. Oncol. 2020, 41, 444–448. [Google Scholar] [CrossRef]
- Chua, T.C.; Pelz, J.O.W.; Kerscher, A.; Morris, D.L.; Esquivel, J. Critical analysis of 33 patients with peritoneal carcinomatosis secondary to colorectal and appendiceal signet ring cell carcinoma. Ann. Surg. Oncol. 2009, 16, 2765–2770. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Oren, N.C.; Kertowidjojo, E.C. Low-grade mucinous neoplasm arising from mature ovarian teratoma with pseudomyxoma peritonei. Int. J. Gynecol. Cancer 2023, 33, 1005–1006. [Google Scholar] [CrossRef]
- Ponzini, F.; Kowal, L.; Ghafoor, M.; Goldberg, A.; Chan, J.; Lamm, R.; Cannaday, S.M.; Richard, S.D.; Nevler, A.; Lavu, H.; et al. Rare occurrence of pseudomyxoma peritonei (PMP) syndrome arising from a malignant transformed ovarian primary mature cystic teratoma treated by cytoreductive surgery and HIPEC: A case report. World J. Surg. Oncol. 2022, 20, 78. [Google Scholar] [CrossRef]
- Csanyi-Bastien, M.; Blanchard, F.; Lamy, A.; Sabourin, J.C. A case of Pseudomyxoma Peritonei of an unexpected origin. Diagn. Pathol. 2021, 16, 119. [Google Scholar] [CrossRef]
- Singh, M. A general overview of mucocele of appendix. J. Family Med. Prim. Care 2020, 9, 5867. [Google Scholar] [CrossRef]
- Ayadi, C.; Naggar, A.; Andour, H.; Chraa, F.Z.; Jerguigue, H.; Latib, R.; Omor, Y. Appendiceal mucocele with pseudomyxoma peritonei mimicking ovarian tumor with peritoneal carcinomatosis. Radiol. Case Rep. 2022, 17, 3000. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Martínez, J.; Palomo-Rodríguez, M.F.; Brenner-Anidjar, R.D.; Márquez-Maraver, F.; Pantoja-Garrido, M.; Gutiérrez-Domingo, Á. Primary pseudomyxoma peritonei originated from ovaries. Ginecol. Obstet. Mex. 2022, 90, 706–712. [Google Scholar] [CrossRef]
- Alghamdi, A.O.; Aldossary, M.Y.; Alsawidan, M.; AlBahar, S. Low grade appendiceal mucinous neoplasm mimicking an ovarian cyst: A case report. Int. J. Surg. Case Rep. 2020, 70, 145–148. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Chang, D. Lymph node positive pseudomyxoma peritonei. Eur. J. Surg. Oncol. 2022, 48, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Pitfalls in Cutaneous Melanoma Lymphatic Drainage—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26988547/ (accessed on 4 March 2024).
- Flicek, K.T.; VanBuren, W.; Dudiak, K.; Lahkman, Y.; Chen, L.W.; Butler, K. Borderline epithelial ovarian tumors: What the radiologist should know. Abdom. Radiol. 2021, 46, 2350–2366. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Rokutan, H.; Oda, K.; Tanikawa, M.; Tanimoto, S.; Sone, K.; Mori, M.; Tsuruga, T.; Kohsaka, S.; Tatsuno, K.; et al. Genetic diagnosis of pseudomyxoma peritonei originating from mucinous borderline tumor inside an ovarian teratoma. BMC Med. Genom. 2022, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Teymoordash, S.N.; Arab, M.; Afsharmoghadam, N.; Nouri, B.; Bozorgan, T.J.; Talayeh, M. A rare case of late recurrence in pseudomyxoma peritonei and advanced stage of borderline mucinous ovarian tumor. J. Obstet. Gynecol. Cancer Res. 2020, 5, 103–109. [Google Scholar] [CrossRef]
- Ryu, J.M.; Jeong, Y.Y.; Choi, Y.S.; Lee, S.J. A rare case of pseudomyxoma peritonei caused by borderline mucinous tumor arising from primary mature cystic ovarian teratoma. Eur. J. Gynaecol. Oncol. 2022, 1, 4. [Google Scholar] [CrossRef]
- Nofal, M.; Al Awayshih, M.M.; Yousef, A.J. Synchronous Colonic and Ovarian Tumors: A Case Report. Am. J. Case Rep. 2019, 20, 655. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Andujar, B.; Valenzuela-Molina, F.; Rufián-Peña, S.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Medina-Fernández, F.J.; Díaz-López, C.; Ortega-Salas, R.; Martínez-López, A.; et al. From the Ronnett to the PSOGI Classification System for Pseudomyxoma Peritonei: A Validation Study. Ann. Surg. Oncol. 2021, 28, 2819–2827. [Google Scholar] [CrossRef]
- Luque-González, P.; Sutil, L.A.; Cabezas-Palacios, M.N.; Jiménez-Gallardo, J.; Rodríguez-Jiménez, I. Peritonei pseudomyxoma diagnosed in the context of a high suspicion of ovarian carcinoma: A case report. Ginecol. Obstet. Mex. 2021, 89, 549–555. [Google Scholar] [CrossRef]
- Arjona-Sánchez, Á.; Martínez-López, A.; Valenzuela-Molina, F.; Rufián-Andújar, B.; Rufián-Peña, S.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Medina-Fernández, F.J.; Díaz-López, C.; et al. A Proposal for Modification of the PSOGI Classification According to the Ki-67 Proliferation Index in Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2022, 29, 126–136. [Google Scholar] [CrossRef]
- Wang, B.; Yao, J.; Ma, R.; Liu, D.; Lu, Y.; Shi, G.; An, L.; Xia, A.; Chen, F.; Pang, S.; et al. The mutational landscape and prognostic indicators of pseudomyxoma peritonei originating from the ovary. Int. J. Cancer 2021, 148, 2036–2047. [Google Scholar] [CrossRef]
- Dundr, P.; Singh, N.; Nožičková, B.; Němejcová, K.; Bártů, M.; Stružinská, I. Primary mucinous ovarian tumors vs. ovarian metastases from gastrointestinal tract, pancreas and biliary tree: A review of current problematics. Diagn. Pathol. 2021, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; El-Sanharawi, I.; Dohan, A.; Sebuhyan, M.; Guedon, A.; Delarue, A.; Boutigny, A.; Mohamedi, N.; Magnan, B.; Kemel, S.; et al. Blood Flow and Shear Stress Allow Monitoring of Progression and Prognosis of Tumor Diseases. Front. Physiol. 2021, 12, 693052. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, R.; Jeuken, J.W.; Bosch, S.L.; van Erning, F.N.; Simkens, L.H.; de Hingh, I.H.; Roumen, R.M. Biomarker concordance between primary colorectal cancer and ovarian metastases: A Dutch cohort study. J. Cancer Res. Clin. Oncol. 2023, 149, 5677–5685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, Y.; Xu, M.; Liu, J. Rapid recurrence of a ruptured mucinous borderline ovarian tumor harboring K-RAS mutation followed by progression into anaplastic carcinoma with TP53 mutation. Heliyon 2022, 8, e10877. [Google Scholar] [CrossRef]
- Fu, F.; Ma, X.; Lu, Y.; Xu, H.; Ma, R. Clinicopathological Characteristics and Prognostic Prediction in Pseudomyxoma Peritonei Originating From Mucinous Ovarian Cancer. Front. Oncol. 2021, 11, 641053. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Gulay, K.C.M.; de Mendoza, T.H.; Tiriac, H.; Baumgartner, J.; Kelly, K.; Veerapong, J.; Lowy, A.M. Culture and Imaging of Ex Vivo Organotypic Pseudomyxoma Peritonei Tumor Slices from Resected Human Tumor Specimens. J. Vis. Exp. 2022, 2022, e64620. [Google Scholar] [CrossRef]
- Fonseca, C.; Carvalho, S.; Cunha, T.M.; Gil, R.T.; Abecasis, N. The many faces of pseudomyxoma peritonei: A radiological review based on 30 cases. Radiol. Bras. 2019, 52, 372–377. [Google Scholar] [CrossRef]
- Campos, N.M.F.; Almeida, V.; Semedo, L.C. Peritoneal disease: Key imaging findings that help in the differential diagnosis. Br. J. Radiol. 2022, 95, 20210346. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.R.; García, N.F.; Salazar, D.J.T.; Illán, R.G.; Sánchez, T.B.D. Imaging findings for mucinous tumors of the abdomen and pelvis. Radiologia 2019, 61, 370–387. [Google Scholar] [CrossRef]
- Noghabaei, G.; Arab, M.; Fazli, G.; Fallah-Talouki, G.; Raoufi, M.; Tahmasebi, H.; Ghavami, B. A Case of Appendiceal Mucocele Mimicking Adnexal Mass in a Young Woman with Chronic Abdominal Pain. J. Obstet. Gynecol. Cancer Res. 2023, 8, 300–304. [Google Scholar] [CrossRef]
- Qiao, J.J.; Yu, J.; Yu, Z.; Li, N.; Song, C.; Li, M. Contrast-Enhanced Ultrasonography in Differential Diagnosis of Benign and Malignant Ovarian Tumors. PLoS ONE 2015, 10, e0118872. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.O.; Sugawara, E.; Tonooka, A.; Saida, T.; Sakata, A.; Fukunaga, Y.; Kanao, H.; Satoh, T.; Noguchi, M.; Terauchi, T. Mucinous tumors arising from ovarian teratomas as another source of pseudomyxoma peritoneii: MR findings comparison with ovarian metastases from appendiceal mucinous tumors. BJR|Open 2023, 5, 20220036. [Google Scholar] [CrossRef] [PubMed]
- Kostov, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Yordanov, A.; Slavcheva, S. Pseudomyxoma peritonei of appendiceal origin mimicking ovarian cancer—A case report with literature review. Menopause Rev./Przegląd Menopauzalny 2021, 20, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Combination of Cancer Antigen 125 and Carcinoembryonic Antigen Can Improve Ovarian Cancer Diagnosis | Ugeskriftet.dk’. Available online: https://ugeskriftet.dk/dmj/combination-cancer-antigen-125-and-carcinoembryonic-antigen-can-improve-ovarian-cancer (accessed on 28 March 2024).
- Radhakrishnan, A.; Malukar, N.; Jain, S. Serum CA-125 and Serum CEA Ratio to Distinguish between Ovarian Malignancies and Non-ovarian Malignancies. Indian J. Med. Biochem. 2020, 24, 97. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Lavie, O.; Auslander, R.; Sagi, S. CEA in evaluation of adnexal mass: Retrospective cohort analysis and review of the literature. Int. J. Biol. Markers 2015, 30, e394–e400. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Hirata, Y.; Mukai, S.; Nishida, T.; Ishikawa, S.; Kai, A.; Kohata, A.; Okimoto, S.; Fujisaki, S.; Fukuda, S.; et al. Multiple peritoneal dissemination of T2 colorectal cancer without lymph node metastases: A case report. J. Surg. Case Rep. 2020, 2020, rjaa118. [Google Scholar] [CrossRef] [PubMed]
- Alsaman, M.Z.B.; Anbar, A.; Nawlo, A.; Almooay, A.; Darwish, A.; Mohammad, M. Incidental diagnosis of pseudomyxoma peritonei by laparoscopy: A rare case from Syria. J. Surg. Case Rep. 2022, 2022, rjac558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, C.; Xu, M.; Wu, X. Appendiceal mucinous neoplasm mimics ovarian tumors: Challenges for preoperative and intraoperative diagnosis and clinical implication. Eur. J. Surg. Oncol. 2019, 45, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Raje, S.; Arvind, S.; Rao, G. A case of Endometrioid endometrial adenocarcinoma with synchronous low-grade Appendiceal mucinous neoplasm and Pseudomyxoma peritonei. J. Minim. Access Surg. 2021, 17, 418–420. [Google Scholar] [CrossRef]
- Tamsin, A.; Schillebeeckx, C.; Van Langenhove, C.; Eeckt, K.V.; Ost, D.; Wetzels, K. Mucinous cystadenocarcinoma in the renal pelvis: Primary or secondary? Case report and literature review. Acta Chir. Belg. 2020, 120, 417–424. [Google Scholar] [CrossRef]
- Taslicay, C.A.; Asadullayeva, M.; Civriz, A.H.; Posteki, G. Benign multicystic peritoneal mesothelioma mimicking mucinous ovarian neoplasm with pseudomyxoma peritonei. BMJ Case Rep. 2023, 16, e254116. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, A. Ruptured primary mucinous cystadenoma of spleen leading to mucinous ascites. BMJ Case Rep. 2019, 12, e231212. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Gohda, Y.; Inagaki, F.; Kataoka, A.; Takemura, N.; Miyazaki, H.; Igari, T.; Kiyomatsu, T.; Yano, H.; Kokudo, N. A case of pseudomyxoma peritonei arising from a perforated intraductal papillary mucinous neoplasm that underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Clin. J. Gastroenterol. 2024, 17, 188–197. [Google Scholar] [CrossRef]
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Nishino, E.; Yonemura, Y. First Report of a Low-Grade Pseudomyxoma peritonei Originating from Gall Bladder. Visc. Med. 2021, 37, 222. [Google Scholar] [CrossRef]
- Eymerit-Morin, C.; Brun, J.L.; Vabret, O.; Devouassoux-Shisheboran, M. Tumeurs frontières de l’ovaire. Recommandations pour la pratique clinique du CNGOF—Biopathologie des tumeurs frontières de l’ovaire. Gynécologie Obs. Fertil. Sénologie 2020, 48, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Hegg, K.S.; Mack, L.A.; Bouchard-Fortier, A.; Temple, W.J.; Gui, X. Macroscopic and microscopic characteristics of low grade appendiceal mucinous neoplasms (LAMN) on appendectomy specimens and correlations with pseudomyxoma peritonei development risk. Ann. Diagn. Pathol. 2020, 48, 151606. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Cascales-Campos, P.; Muñoz-Casares, C.; Asensio, E.; Torres-Melero, J.; Prada-Villaverde, A.; Caravaca-García, I.; Gutiérrez-Calvo, A.; Vaqué, J.; et al. Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). J. Clin. Med. 2023, 12, 3774. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, V.; Warrier, S.; Michael, M.; McCormick, J.; Ramsay, R.; Lynch, C.; Heriot, A. Perceptions in the management of colorectal peritoneal metastases: A bi-national survey of colorectal surgeons. Pleura Peritoneum 2019, 4, 20190022. [Google Scholar] [CrossRef]
- Bartoška, P.; Antoš, F.; Vítek, P.; Marx, J.; Kopic, J.; Holečková, P. Pseudomyxoma Peritonei. Klin. Onkol. 2019, 32, 329–332. [Google Scholar] [CrossRef]
- Bartoška, P.; Antoš, F.; Vítek, P.; Marx, J.; Holečková, P.; Novotný, M.; Kengbaeva, M. Pseudomyxoma peritonei (PMP) and its therapy—20 years experience of a single surgical department. Rozhl. Chir. 2020, 99, 159–166. [Google Scholar] [CrossRef]
- Mercier, F.; Dagbert, F.; Pocard, M.; Goéré, D.; Quenet, F.; Wernert, R.; Dumont, F.; Brigand, C.; Passot, G.; Glehen, O. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open 2018, 3, 195–202. [Google Scholar] [CrossRef]
- Conley, A.B.; Fournier, K.F.; Sood, A.K.; Frumovitz, M. Secondary Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Advanced or Recurrent Mucinous Ovarian Cancer. Obstet. Gynecol. 2023, 141, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Antoš, F.; Bartoška, P.; Vobořil, R. Peritoneal surface malignancy spread reoperations after cytoreductive surgery + HIPEC. Rozhl. Chir. 2021, 100, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.; Ray, M.; Bansal, B.; Bhoriwal, S.; Bhatnagar, S.; Garg, R.; Gupta, N.; Sharma, A.; Kumar, L.; Thulkar, S.; et al. Feasibility and outcomes of cytoreductive surgery and HIPEC for peritoneal surface malignancies in low- and middle-income countries: A single-center experience of 232 cases. World J. Surg. Oncol. 2021, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Souadka, A.; Essangri, H.; Majbar, M.A.; Benkabbou, A.; Boutayeb, S.; Amrani, L.; Ghannam, A.; El Ahmadi, B.; Belkhadir, Z.H.; Mohsine, R.; et al. Mid-term audit of a national peritoneal surface malignancy program implementation in a low middle income country: The moroccan experience. Cancers 2021, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sanchez, A.; Aziz, O.; Passot, G.; Salti, G.; Esquivel, J.; Van der Speeten, K.; Piso, P.; Nedelcut, D.S.; Sommariva, A.; Yonemura, Y.; et al. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for limited peritoneal metastasis. The PSOGI international collaborative registry. Eur. J. Surg. Oncol. 2021, 47, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Psomiadou, V.; Fotiou, A.; Prodromidou, A.; Iavazzo, C. Is minimal invasive surgical treatment of ovarian cancer plus HIPEC a utopia? A review of the literature. Eur. J. Gynaecol. Oncol. 2021, 42, 1001–1005. [Google Scholar] [CrossRef]
- Kitai, T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: A systematic review including evidence from Japan. Surg. Today 2021, 51, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Robella, M.; Vaira, M.; Cinquegrana, A.; de Simone, M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Morbidity and postoperative outcomes. Minerva Chir. 2019, 74, 195–202. [Google Scholar] [CrossRef]
- Naved, S.A.; Parpia, S.S.; Ali, H.S. Development of stress-induced cardiomyopathy after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Pak. Med. Assoc. 2021, 71, 1686–1688. [Google Scholar] [CrossRef]
- Smibert, O.C.; Slavin, M.A.; Teh, B.; Heriot, A.G.; Penno, J.; Ismail, H.; Thursky, K.A.; Worth, L.J. Epidemiology and risks for infection following cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Support. Care Cancer 2020, 28, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Pintado, M.C.; Lasa Unzúe, I.; Gómez Sanz, R.; Diez Alonso, M.; Ortega, M.A.; Álvarez de Mon, M.; Nevado Losada, E.; Gutierrez Calvo, A. Hematological Alterations after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Med. 2023, 12, 4323. [Google Scholar] [CrossRef] [PubMed]
- Rubio-López, J.D.; Durán-Martínez, M.; Moreno-Blázquez, A.; Rodríguez-Ortiz, L.; Rufián-Andújar, B.; Valenzuela-Molina, F.; Adam, Á.C.; Sánchez-Hidalgo, J.M.; Rufián-Peña, S.; Romero-Ruiz, A.; et al. Intraoperative metabolic changes associated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Langenbecks Arch. Surg. 2024, 408, 34. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.; Manatakis, D.K.; Papakonstantinou, K.; Kyriazanos, I.D. A comprehensive review of childbearing after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Arch. Gynecol. Obstet. 2020, 302, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Antomarchi, O.; Canis, M.; Bourdel, N.; Chauvet, P. Management of peritoneal pseudomyxoma in pregnant women: A case report and review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102351. [Google Scholar] [CrossRef] [PubMed]
- Aburahmah, M.; Hijji, T.M.; Saif, L.T.; Kalagi, D.; Azzam, A.Z.; Amin, T. Feasibility of combining oncology surgery with bariatric surgery; a two-patient case series of sleeve gastrectomy with cytoreductive surgery and HIPEC. J. Surg. Case Rep. 2022, 2022, rjab588. [Google Scholar] [CrossRef] [PubMed]
- Hishida, T.; Masai, K.; Kaseda, K.; Asakura, K.; Asamura, H. Debulking surgery for malignant tumors: The current status, evidence and future perspectives. Jpn. J. Clin. Oncol. 2021, 51, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Moran, B.J. Time to Evolve Terminology from “Debulking” to Cytoreductive Surgery (CRS) in Ovarian Cancer. Ann. Surg. Oncol. 2021, 28, 5805–5807. [Google Scholar] [CrossRef] [PubMed]
- Nors, J.; Iversen, L.H.; Nielsen, K.; Sørensen, M.M.; Verwaal, V.J.; Funder, J.A. Peritoneal metastases found in routinely resected specimens after cytoreductive surgery and heated intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2022, 48, 795–802. [Google Scholar] [CrossRef]
- Saadeh, R.; Berthet, A.; Marchant, S.; Kraus, F.; Aissaoui, O.; Narducci, F.; Leblanc, E.; Hudry, D. Total parietal peritonectomy for 61 patients: A retrospective study. Eur. J. Gynaecol. Oncol. 2022, 43, 17–25. [Google Scholar] [CrossRef]
- Hiraide, S.; Komine, K.; Sato, Y.; Ouchi, K.; Imai, H.; Saijo, K.; Takahashi, M.; Takahashi, S.; Shirota, H.; Takahashi, M.; et al. Efficacy of modified FOLFOX6 chemotherapy for patients with unresectable pseudomyxoma peritonei. Int. J. Clin. Oncol. 2020, 25, 774–781. [Google Scholar] [CrossRef]
- Yazawa, R.; Yazawa, H.; Fukuda, K.; Ohara, M.; Osuka, F. Four cases of pseudomyxoma peritonei with ovarian tumors at our hospital. Fukushima J. Med. Sci. 2023, 69, 57–65. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hübner, M.; Alyami, M.; Eveno, C.; Gagnière, J.; Pache, B.; Pocard, M.; Bakrin, N.; Quénet, F. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: A multicenter study. Eur. J. Surg. Oncol. 2019, 45, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Ceribelli, C.; Debs, T.; Chevallier, A.; Piche, M.A.; Bereder, J.M. Initial experience of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a French hyperthermic intraperitoneal chemotherapy (HIPEC) expert center. Surg. Endosc. 2020, 34, 2803–2806. [Google Scholar] [CrossRef]
- Rana, A.A.; Rana, A.; Hewett, P. Multiple Small Bowel Perforations Secondary to Tumor Lysis—A Complication of Pseudomyxoma Peritonei in a Patient Undergoing Intraperitoneal Chemotherapy. J. Gastrointest. Cancer 2020, 51, 289–291. [Google Scholar] [CrossRef]
- Guchelaar, N.A.D.; Noordman, B.J.; Koolen, S.L.W.; Mostert, B.; Madsen, E.V.E.; Burger, J.W.A.; Brandt-Kerkhof, A.R.M.; Creemers, G.J.; de Hingh, I.H.J.T.; Luyer, M.; et al. Intraperitoneal Chemotherapy for Unresectable Peritoneal Surface Malignancies. Drugs 2023, 83, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Mageed, H.A.; Van Der Speeten, K.; Sugarbaker, P. The many faces of intraperitoneal chemotherapy. Surg. Oncol. 2022, 40, 101676. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Shi, X.L.; Wang, Y.F.; Yang, F.; Wang, T.T.; Peng, C.X. Apatinib for treatment of a pseudomyxoma peritonei patient after surgical treatment and hyperthermic intraperitoneal chemotherapy: A case report. World J. Clin. Cases 2019, 7, 3881–3886. [Google Scholar] [CrossRef]
- Borg, P.; Ng, H.H.L.; Mullan, D.; Aziz, O.; Laasch, H.U. Ultrasound-guided day-case wide-bore percutaneous mucin aspiration in advanced pseudomyxoma peritonei. Clin. Radiol. 2023, 78, e458–e462. [Google Scholar] [CrossRef]
- Sullivan, B.J.; Bolton, N.; Sarpel, U.; Magge, D. A unique presentation of superinfected pseudomyxoma peritonei secondary to a low-grade appendiceal mucinous neoplasm. World J. Surg. Oncol. 2019, 17, 34. [Google Scholar] [CrossRef]
- López-Basave, H.N.; Morales-Vázquez, F.; Herrera-Gómez, Á.; Miranda-Devora, G.; Padilla-Rosciano, A.E.; Castillo-Morales, C.; Paleta-Torres, C.A.; Vázquez-Cortes, E. Peritoneal carcinomatosis index and overall survival in patients taken to cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Cirugía Cir. 2023, 91, 195–199. [Google Scholar] [CrossRef]



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, S.; Marincas, M.; Madge, O.L.; Dicu-Andreescu, I.G.; Chitoran, E.; Rotaru, V.; Cirimbei, C.; Gherghe, M.; Ene, A.; Rosca, R.; et al. Ovarian Causes of Pseudomyxoma Peritonei (PMP)—A Literature Review. Cancers 2024, 16, 1446. https://doi.org/10.3390/cancers16081446
Ionescu S, Marincas M, Madge OL, Dicu-Andreescu IG, Chitoran E, Rotaru V, Cirimbei C, Gherghe M, Ene A, Rosca R, et al. Ovarian Causes of Pseudomyxoma Peritonei (PMP)—A Literature Review. Cancers. 2024; 16(8):1446. https://doi.org/10.3390/cancers16081446
Chicago/Turabian StyleIonescu, Sinziana, Marian Marincas, Octavia Luciana Madge, Irinel Gabriel Dicu-Andreescu, Elena Chitoran, Vlad Rotaru, Ciprian Cirimbei, Mirela Gherghe, Adina Ene, Robert Rosca, and et al. 2024. "Ovarian Causes of Pseudomyxoma Peritonei (PMP)—A Literature Review" Cancers 16, no. 8: 1446. https://doi.org/10.3390/cancers16081446
APA StyleIonescu, S., Marincas, M., Madge, O. L., Dicu-Andreescu, I. G., Chitoran, E., Rotaru, V., Cirimbei, C., Gherghe, M., Ene, A., Rosca, R., Radu, M., & Simion, L. (2024). Ovarian Causes of Pseudomyxoma Peritonei (PMP)—A Literature Review. Cancers, 16(8), 1446. https://doi.org/10.3390/cancers16081446