Effects of Adjuvant Exercise and Nutrition Therapy on Muscle Fibre Biomechanics in Gastrointestinal Cancer Patients
Abstract
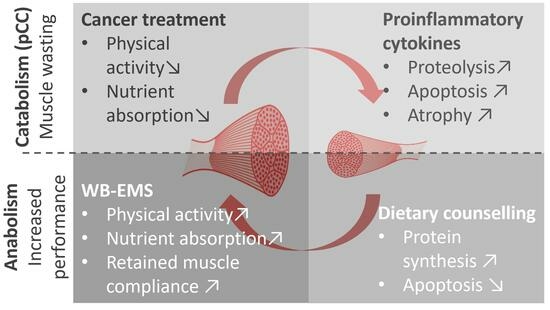
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Being in other nutrition or exercise intervention studies;
- Study-independent exercise ≥ once a week;
- Ingestion of anabolic or dietary supplements;
- Occurrence of heavy cardiovascular events;
- Epilepsy;
- Severe neurological disorders;
- Skin lesions around the electrodes;
- Conductive materials or electronical implants in the body;
- Pregnancy;
- Chronic diseases (e.g., HIV or Hepatitis C/D/E).
2.2. Study Design and Multi-Modal Therapy
- Inflammation (C-reactive protein, CRP, normal value < 5 mg/L);
- Nutrition (albumin, 35–55 g/L);
- Renal function (creatinine, 0.51–1.17 mg/dL);
- Haematological parameters (leucocytes, 4.4–11.3 × 103/μL; thrombocytes, 150–300 × 103/μL; erythrocytes, 4.1–6.0 × 106/μL; haematocrit, 35–48%; and haemoglobin, 11.5–18.0 g/dL).
2.3. Biopsy and Sample Processing
2.4. Physiological Solutions
- High-activating solution (HA): Ca2+-saturated environment to chemically induce maximum force generation ([Ca2+]free~12 μM).
- High-relaxing solution (HR): strong Ca2+-chelating (EGTA) environment to buffer excess Ca2+.
- Low-relaxing solution (LR): the high Ca2+-chelating EGTA was exchanged for the low-affinity HDTA prior to any subsequent solution exposure.
- Loading solution (LS): the previously emptied SR was re-loaded for a defined time (consisting of HA and HR titrated to [Ca2+]free~300 nM).
- Release solution (RS): a total of 30 mM caffeine was added to LR, which triggered SR Ca2+ release. The force transient was proportional to the releasable SR Ca2+ content.
- pCa solutions: the Ca2+ sensitivity of the contractile apparatus was assessed for defined pCa values (mixture of HA and HR; calculated using React (Geoffrey Lee, University of Glasgow).
2.5. Active and Passive Biomechanics Recordings with the MyoRobot
- Caffeine-induced, Ca2+-mediated force transients: The fibre was exposed to HR to wash off saponin and excess Ca2+ buffer before exposure to LR. It was then submerged in LS for 90 s to load the SR. The caffeine-induced force transient was triggered in caffeine-rich RS for 60 s. Eventually, the maximum force was triggered in Ca2+-saturated HA solution for 10 s (see Figure 3A,B).
- Ca2+ sensitivity of the contractile apparatus: the specimen was consecutively exposed to solutions of increasing Ca2+ concentrations (decreasing pCa (−log10[Ca2+])) for a duration of 10 s each (see Figure 4A).
- Passive axial stiffness/compliance: the muscle fibre was stretched at 0.44 μm/s in the LR solution to 140% L0 (see Figure 5A).
2.6. Data Analysis and Statistics
- Caffeine-induced, SR Ca2+-release force transients: Force data were read-in and corrected for their baseline (force in HR solution). The plateau force was determined by the 99% quantile. The force ratio of the Ca2+-release peak force to maximum Ca2+-saturated force was calculated (see Figure 3A).
- Ca2+ sensitivity of the contractile apparatus: The 99% quantile was used to determine the plateau forces at each pCa step (Figure 4B). The forces were normalised to the maximum force and plotted against the pCa values. A four-parameter Hill equation () was fitted to the data, where a = 1 and y0 = 0. The Hill coefficient (b) and the pCa50 value (−log10([c]) were utilised to reconstruct a mean fit to the average data points (Figure 4B).
- Passive axial stiffness/compliance: At 140% L0, the maximum passive restoration force was determined. Linear fits were applied to every section of 10% stretch. The slope represents the passive axial stiffness. Its inverse is the fibre’s axial compliance (see Figure 5A).
3. Results
3.1. Study Design and Patient Data
3.2. Maximum Ca2+-Saturated Force and Caffeine-induced, SR Ca2+-Release Force Are Compromised in (Pre-)CC Patients but Ameliorated by Adjuvant Multi-Modal Therapy
3.3. Ca2+ Sensitivity of Rectus Abdominis Single Fibres Seems Unaffected in Patients with or without Adjuvant Multi-Modal Therapy
3.4. Passive Axial Single Muscle Fibre Stiffness Is Increased in (Pre-)CC Patients without Multi-Modal Adjuvant Treatment
4. Discussion
4.1. Compromised SR Ca2+-Release-Induced Force and Maximum Force Are Ameliorated in (Pre-)CC Patients Receiving Adjuvant Multi-Modal Therapy
4.2. Unaltered Ca2+ Sensitivity in (Pre-)CC Patients Suggests Unaltered Quality of Contractility
4.3. Increased Passive Stiffness in Single Muscle Fibres from (Pre-)CC Patients Receiving No Multi-Modal Adjuvant Intervention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Anker, S.D.; Coats, A.J.S.; von Haehling, S. The Journal of Cachexia, Sarcopenia and Muscle stays the front-runner in geriatrics and gerontology. J. Cachexia Sarcopenia Muscle 2019, 10, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Bossola, M.; Aversa, Z.; Bellantone, R.; Rossi Fanelli, F. Prevention and treatment of cancer cachexia: New insights into an old problem. Eur. J. Cancer 2006, 42, 31–41. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachexia Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- van Doren, B.A.; Odum, S.M.; Mason, J.B.; Arthur, S. Cachexia Signficantly Increases the Risk of Major Peri-Operative Complications and In-Hospital Mortality in Total Joint Replacement Patients: Results of a Matched Cohort Study. Value Health 2016, 19, A224. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.; Ruan, G.T.; Zhang, K.P.; Tang, M.; Zhang, Q.; Song, M.M.; Zhang, X.W.; Ge, Y.Z.; Yang, M.; et al. One-Year Mortality in Patients with Cancer Cachexia: Association with Albumin and Total Protein. Cancer Manag. Res. 2021, 13, 6775–6783. [Google Scholar] [CrossRef]
- Isaac, S.T.; Tan, T.C.; Polly, P. Endoplasmic Reticulum Stress, Calcium Dysregulation and Altered Protein Translation: Intersection of Processes That Contribute to Cancer Cachexia Induced Skeletal Muscle Wasting. Curr. Drug Targets 2016, 17, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef]
- Dhanapal, R.; Saraswathi, T.; Govind, R.N. Cancer cachexia. J. Oral Maxillofac. Pathol. 2011, 15, 257–260. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bertz, H.; Bischoff, S.C.; Fietkau, R.; Herrmann, H.J.; Holm, E.; Horneber, M.; Hütterer, E.; Körber, J.; Schmid, I.; et al. S3-Leitline der Deutschen Gesellschaft für Ernährungsmedizin e. V. (DGEM) in Kooperation mit der Deutschen Gesellschaft für Hämatologie und Onkologie e. V. (DGHO), der Arbeitsgemeinschaft “Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin“ der Deutschen Krebsgesellschaft (ASORS) und der Österreichischen Arbeitsgemeinschaft für klinische Ernährung (AKE). Aktuel. Ernahrungsmed. 2015, 40, e1–e74. [Google Scholar] [CrossRef]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Friedrich, O.; Haug, M.; Reischl, B.; Prölß, G.; Kiriaev, L.; Head, S.I.; Reid, M.B. Single muscle fibre biomechanics and biomechatronics—The challenges, the pitfalls and the future. Int. J. Biochem. Cell Biol. 2019, 114, 105563. [Google Scholar] [CrossRef]
- Banduseela, V.; Ochala, J.; Lamberg, K.; Kalimo, H.; Larsson, L. Muscle paralysis and myosin loss in a patient with cancer cachexia. Acta Myol. 2007, 26, 136–144. [Google Scholar] [PubMed]
- Ochala, J.; Larsson, L. Effects of a preferential myosin loss on Ca2+ activation of force generation in single human skeletal muscle fibres. Exp. Physiol. 2008, 93, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Haug, M.; Meyer, C.; Reischl, B.; Prölß, G.; Nübler, S.; Schürmann, S.; Schneidereit, D.; Heckel, M.; Pöschel, T.; Rupitsch, S.J.; et al. MyoRobot 2.0: An advanced biomechatronics platform for automated, environmentally controlled skeletal muscle single fiber biomechanics assessment employing inbuilt real-time optical imaging. Biosens. Bioelectron. 2019, 138, 111284. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Haug, M.; Reischl, B.; Prölß, G.; Pollmann, C.; Buckert, T.; Keidel, C.; Schürmann, S.; Hock, M.; Rupitsch, S.; Heckel, M.; et al. The MyoRobot: A novel automated biomechatronics system to assess voltage/Ca2+ biosensors and active/passive biomechanics in muscle and biomaterials. Biosens. Bioelectron. 2018, 102, 589–599. [Google Scholar] [CrossRef]
- Head, S.I. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp. Physiol. 2010, 95, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Mariani, L. Defining and classifying cancer cachexia: A proposal by the SCRINIO Working Group. JPEN J. Parenter. Enteral Nutr. 2009, 33, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Prokopchuk, O.; Esefeld, K.; Gröschel, S.; Bachmann, J.; Lorenzen, S.; Friess, H.; Halle, M.; Martignoni, M.E. The clinical picture of cachexia: A mosaic of different parameters (experience of 503 patients). BMC Cancer 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Illman, J.; Corringham, R.; Robinson, D., Jr.; Davis, H.M.; Rossi, J.F.; Cella, D.; Trikha, M. Are inflammatory cytokines the common link between cancer-associated cachexia and depression? J. Support. Oncol. 2005, 3, 37–50. [Google Scholar] [PubMed]
- Shum, A.M.Y.; Poljak, A.; Bentley, N.L.; Turner, N.; Tan, T.C.; Polly, P. Proteomic profiling of skeletal and cardiac muscle in cancer cachexia: Alterations in sarcomeric and mitochondrial protein expression. Oncotarget 2018, 9, 22001–22022. [Google Scholar] [CrossRef] [PubMed]
- van de Worp, W.R.P.H.; Schools, A.M.W.J.; Theys, J.; Van Helvoort, A.; Langen, R.C. Nutritional Interventions in Cancer Cachexia: Evidence and Perspectives from Experimental Models. Front. Nutr. 2020, 7, 601329. [Google Scholar] [CrossRef] [PubMed]
- Taskin, S.; Stumpf, V.I.; Bachmann, J.; Weber, C.; Martignoni, M.R.; Friedrich, O. Motor protein function in skeletal abdominal muscle of cachectic cancer patients. J. Cell. Mol. Med. 2014, 18, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.M.; Nosacka, R.L.; Delitto, D.; Gerber, M.H.; Cameron, M.E.; Trevino, J.G.; Judge, A.R. Skeletal Muscle Fibrosis in Pancreatic Cancer Patients with Respect to Survival. JNCI Cancer Spectr. 2018, 2, pky043. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Oliveira, C.C.; Busquets, S.; Toledo, M.; Penna, F.; Aylwin, M.P.; Sirisi, S.; Silva, A.P.; Orpí, M.; García, A.; Sette, A.; et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: Altered energetic efficiency? Biochim. Biophys. Acta 2013, 1830, 2770–2778. [Google Scholar] [CrossRef]
- Murphy, R.M.; Larkins, N.T.; Mollica, J.P.; Beard, N.A.; Lamb, G.D. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 2009, 587, 443–460. [Google Scholar] [CrossRef]
- Rossi, A.E.; Boncompagni, S.; Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef]
- Martin, L.; Muscaritoli, M.; Bourdel-Marchasson, I.; Kubrak, C.; Laird, B.; Gagnon, B.; Chasen, M.; Gioulbasanis, I.; Wallengren, O.; Voss, A.C.; et al. Diagnostic criteria for cancer cachexia: Reduced food intake and inflammation predict weight loss and survival in an international, multi-cohort analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1189–1202. [Google Scholar] [CrossRef]
- Schmitt, T.L.; Martignoni, M.E.; Bachmann, J.; Fechtner, K.; Friess, H.; Kinscherf, R.; Hildebrandt, W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 2007, 85, 647–654. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015, 79, 91–108. [Google Scholar] [CrossRef]
- Micheo, W.; Baerga, L.; Miranda, G. Basic principles regarding strength, flexibility, and stability exercises. PM&R 2012, 4, 805–811. [Google Scholar] [CrossRef]
- Zopf, Y.; Herrmann, H.J. Grundlagen der Kachexie bei Tumorpatienten. Onkologe 2016, 22, 233–240. [Google Scholar] [CrossRef]
- Michael, M.; Kovbasyuk, L.; Ritter, P.; Reid, M.B.; Friedrich, O.; Haug, M. Redox Balance Differentially Affects Biomechanics in Permeabilized Single Muscle Fibres-Active and Passive Force Assessments with the Myorobot. Cells 2022, 11, 3715. [Google Scholar] [CrossRef]





| Study Groups | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | G1 Non-Tumour (n = 5) | G2 Tumour (n = 9) | G3 Tumour with WB-EMS/Nutrition (n = 3) | |||||
| Pre | Post | G1 vs. G2 | G1 vs. G3 | G2 vs. G3 | G3 Pre vs. Post | |||
| Sex | - | - | - | - | ||||
| Male, n (%) | 3 (60%) | 7 (77.8%) | 1 (33.3%) | - | ||||
| Female, n (%) | 2 (40%) | 2 (22.2%) | 2 (66.6%) | - | ||||
| Age (y) | 61.2 ± 10.0 | 62.3 ± 8.0 | 62.7 ± 14.1 | - | 0.976 a | 0.976 a | 0.999 a | - |
| Tumour, stage (UICC) | - | - | - | - | ||||
| I, n (%) | - | 2 (22.2%) | 1 (33.3%) | - | ||||
| II, n (%) | - | 1 (11.1%) | 1 (33.3%) | - | ||||
| III, n (%) | - | 4 (44.4%) | 1 (33.3%) | - | ||||
| IV, n (%) | - | 2 (22.2%) | 0 (0%) | - | ||||
| Oncological therapy | - | - | - | - | ||||
| Chemotherapy, n (%) | - | 2 (22.2%) | 2 (66.7%) e | - | ||||
| Chemo- and radiotherapy, n (%) | - | 5 (55.6%) | 1 (33.3%) e | - | ||||
| No therapy, n (%) | - | 2 (22.2%) | 0 (0%) e | - | ||||
| Body parameters | ||||||||
| Body weight (kg) | 75.5 ± 15.6 | 73.4 ± 7.7 (n = 8) | 83.8 ± 17.1 | 85.8 ± 18.1 | 0.956 a | 0.516 a | 0.343 a | 0.072 c |
| Weight loss in last 6 months (%) | 0 ± 0 | 10.1 ± 9.1 (n = 7) | 3.3 ± 5.7 | - | 0.049 b | >0.999 b | 0.575 b | - |
| Body mass index (kg/m2) | 26.0 ± 2.6 | 24.6 ± 1.9 (n = 8) | 27.9 ± 4.4 | 28 ± 4.6 | 0.641 a | 0.453 a | 0.127 a | 0.070 c |
| Blood parameters | ||||||||
| Albumin (g/L) | 42.2 ± 5.4 | 30.8 ± 6.0 (n = 7) | 39.8 ± 2.7 | 30.4 ± 2.5 | 0.011 a | 0.013 a | 0.81 a | 0.08 c |
| C-reactive protein (mg/L) | 5.6 ± 7.1 | 42.4 ± 63.2 (n = 8) | 4.8 ± 4.8 | 59.3 ± 92.5 | 0.534 a | 0.448 a | 0.907 a | 0.421 c |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.74 ± 0.2 | 0.464 a | 0.546 a | 0.986 a | 0.274 c |
| Haematocrit (%) | 41.6 ± 3.5 | 35.4 ± 5.3 | 39.7 ± 1.9 | 35.3 ± 6.5 | 0.102 a | 0.199 a | 0.99 a | 0.312 c |
| Haemoglobin (g/dL) | 13.9 ± 1.3 | 11.8 ± 1.7 | 13.0 ± 0.6 | 11.6 ± 2.2 | 0.091 a | 0.162 a | 0.973 a | 0.37 c |
| Leucocytes (×103/µL) | 8.2 ± 2.0 | 6.8 ± 1.5 | 8.7 ± 4.2 | 8.9 ± 4.4 | 0.701 b | >0.999 b | >0.999 b | >0.999 d |
| Erythrocytes (×106/µL) | 4.9 ± 0.4 | 4.0 ± 0.7 | 4.7 ± 0.2 | 3.9 ± 0.8 | 0.078 b | 0.146 b | >0.999 b | 0.25 d |
| Thrombocytes (×103/µL) | 341.8 ± 88.0 | 288.8 ± 100.5 | 187.3 ± 54.0 | 183.3 ± 49.6 | 0.943 b | 0.063 b | 0.276 b | 0.75 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haug, M.; Schwappacher, R.; Pollmann, C.; Ritter, P.; Michael, M.; Hermann, H.J.; Grützmann, R.; Mittelstädt, A.; Neurath, M.F.; Zopf, Y.; et al. Effects of Adjuvant Exercise and Nutrition Therapy on Muscle Fibre Biomechanics in Gastrointestinal Cancer Patients. Cancers 2024, 16, 1608. https://doi.org/10.3390/cancers16081608
Haug M, Schwappacher R, Pollmann C, Ritter P, Michael M, Hermann HJ, Grützmann R, Mittelstädt A, Neurath MF, Zopf Y, et al. Effects of Adjuvant Exercise and Nutrition Therapy on Muscle Fibre Biomechanics in Gastrointestinal Cancer Patients. Cancers. 2024; 16(8):1608. https://doi.org/10.3390/cancers16081608
Chicago/Turabian StyleHaug, Michael, Raphaela Schwappacher, Charlotte Pollmann, Paul Ritter, Mena Michael, Hans Joachim Hermann, Robert Grützmann, Anke Mittelstädt, Markus Friedrich Neurath, Yurdagül Zopf, and et al. 2024. "Effects of Adjuvant Exercise and Nutrition Therapy on Muscle Fibre Biomechanics in Gastrointestinal Cancer Patients" Cancers 16, no. 8: 1608. https://doi.org/10.3390/cancers16081608
APA StyleHaug, M., Schwappacher, R., Pollmann, C., Ritter, P., Michael, M., Hermann, H. J., Grützmann, R., Mittelstädt, A., Neurath, M. F., Zopf, Y., & Friedrich, O. (2024). Effects of Adjuvant Exercise and Nutrition Therapy on Muscle Fibre Biomechanics in Gastrointestinal Cancer Patients. Cancers, 16(8), 1608. https://doi.org/10.3390/cancers16081608