YKL-40—A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients?
Abstract
:1. Introduction
2. YKL-40 Gene and Protein
3. YKL-40 in Healthy Subjects
3.1. Tissue

3.2. Plasma Concentrations of YKL-40 in Healthy Subjects
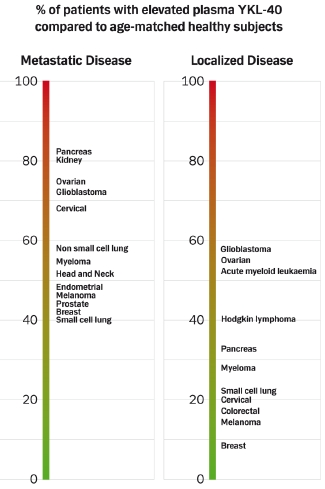
4. YKL-40 in Different Types of Cancer

4.1. Adenocarcinoma
4.1.1. Tissue

4.1.2. Screening
4.1.3. Prevalence of High Plasma YKL-40 Levels

4.1.4. Prognosis
| Diagnosis | Number | Treatment | HR (95% CI) | P-value | Ref. |
|---|---|---|---|---|---|
| Primary breast cancer | 271 | Surgery + Chemotherapy | 1.8 (1.0–3.1) | 0.04 | 49 |
| Metastatic breast cancer | 100 | Chemotherapy | 2.6 (1.6–4.1) | 0.0002 | 48 |
| Colorectal cancer | 603 | Surgery | 1.4 (1.1–1.8) | 0.007 | 52 |
| Metastatic colorectal cancer | 155 | Chemotherapy + Biologics | 1.2 (1.1–1.4) | 0.0045 | 54 |
| Ovarian cancer, stage III | 47 | Surgery + Chemotherapy | 4.0 (1.5–10.3) | 0.005 | 61 |
| Ovarian cancer, 1. relapse | 73 | Chemotherapy | 2.3 (1.3–4.1) | 0.006 | 62 |
| Ovarian cancer, stage I–IV | 76 | Surgery + Chemotherapy | 2.1 (1.4–3.2) | 0.0004 | 36 |
| Metastatic prostate cancer | 150 | Endocrine therapy | 1.3 (1.0–1.7) | 0.02 | 58 |
| Renal cancer | 58 | IL-2 | 3.0 (1.2–7.0) | 0.014 | 72 |
| Small cell lung cancer | 131 | Chemotherapy | 1.9 (1.1–3.4) | 0.02 | 71 |
| Metastatic NSCLC | 189 | Chemotherapy | 1.5¤ | 0.0002 | 56 |
| Cervical, adenocarcinoma | 37 | Surgery or Chemoradiation | 11 (1.3–97) § | 0.03 | 66 |
| Head- and neck cancer | 138 | Radiotherapy | 2.2 (1.4–3.4) | 0.0006 | 68 |
| Melanoma I–II | 234 | Surgery | 3.6 (1.7–7.7) | 0.001 | 74 |
| Melanoma IIB–III* | 147 | Surgery | 1.8 (1.2–2.7) | 0.008 | 75 |
| Melanoma IV | 110 | IL-2, IFN | 1.9 (1.2–2.8) | 0.004 | 73 |
| Acute myeloid leukemia | 78 | Chemotherapy | 1.4 (1.1–1.7) | 0.0002 | 99 |
| Anaplastic astrocytoma | 38 | Surgery | 2.2 (1.0–4.9) | 0.05 | 94 |
| Glioblastoma | 75 | Surgery | 1.4 (1.1–1.9) | 0.02 | 94 |
4.1.5. Treatment Response
4.1.6. Monitoring
4.2. Squamous Cell Carcinoma
4.2.1. Tissue
4.2.2. Prognosis, Treatment Response, and Monitoring
4.3. Small Cell Lung Carcinoma
4.3.1. Tissue
4.3.2. Prognosis, Treatment Response, and Monitoring
4.4. Renal Cell Carcinoma
4.4.1. Tissue
4.4.2. Prognosis, Treatment Response, and Monitoring
4.5. Melanoma
4.5.1. Tissue
4.5.2. Prognosis, Treatment Response, and Monitoring
4.6. Glioblastoma
4.6.1. Tissue
4.6.2. Prognosis, Treatment Response, and Monitoring
4.7. Hematological Malignancies
4.7.1. Tissue
4.7.2. Prognosis, Treatment Response, and Monitoring
4.8. Cancer Stem Cells
4.8.1. Tissue
5. Plasma YKL-40 in Patients with Non-Malignant Diseases Characterized by Inflammation, Tissue Remodeling and Fibrosis
5.1. Inflammatory Diseases
5.1.1. Infectious Diseases
5.1.2. Cardiovascular Diseases
5.1.3. Diabetes
5.1.4. Rheumatic Diseases
5.1.5. Lung Diseases
5.1.6. Inflammatory Bowel Diseases
5.2. Liver Fibrosis
6. What Is the Function of YKL-40?
6.1. Single Nucleotide Polymorphism (SNP)
6.2. Growth Factor
6.3. Protection Factor
6.4. Angiogenesis
6.5. Inflammation
6.6. Tissue Remodeling and Development of Fibrosis
7. Plasma YKL-40—A New Cancer Biomarker?
- Can the clinician measure the biomarker and is the method specific, sensitive, fast and cheap?
- Does the biomarker level add new information of the disease?
- Does the biomarker level help the clinician to treat patients?
| Tumor specific? |
| No—YKL-40 is also produced by non-malignant cells, e.g., inflammatory cells. |
| High specificity for cancer? |
| No—Plasma YKL-40 is also elevated in patients with diseases characterized by acute or chronic inflammation, tissue remodeling and fibrosis; i.e., co-morbidity shall always be considered in cancer patients with high plasma YKL-40. |
| High sensitivity for cancer? |
| No—Plasma YKL-40 is only elevated in a subgroup of cancer patients. |
| Useful for screening? |
| ???—Plasma YKL-40 is elevated many years before a gastrointestinal cancer is diagnosed; more studies are needed. |
| Reflect poor prognosis? |
| Yes—High plasma YKL-40 reflects poor prognosis and is independent of other routinely used biomarkers. |
| Predictor of treatment response? |
| ???—High plasma YKL-40 reflects poor treatment response in some patients; more studies are needed. |
| Useful for monitoring? |
| ???—High plasma YKL-40 may reflects disease progression; more studies are needed. |
8. Future
- Is plasma YKL-40 a useful clinical biomarker in patients with cancer?
- Is YKL-40 a target for development of new cancer therapeutics?
Acknowledgements
References
- Siena, S.; Sartore-Bianchi, A.S.; Di Nicolantonio, F.; Balfour, J.; Bardelli, A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J. Natl. Cancer Inst. 2009, 101, 1308–1324. [Google Scholar] [CrossRef]
- Atkinson, A.J., Jr. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Rehli, M.; Krause, S.W.; Andreesen, R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997, 43, 221–225. [Google Scholar] [CrossRef]
- Rehli, M.; Niller, H.H.; Ammon, C.; Langmann, S.; Schwarzfischer, L.; Andreesen, R.; Krause, S.W. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003, 278, 44058–44067. [Google Scholar]
- Johansen, J.S.; Høyer, P.E.; Larsen, L.A.; Price, P.A.; Møllgård, K. YKL-40 protein expression in the early developing human musculoskeletal system. J. Histochem. Cytochem. 2007, 55, 1213–1228. [Google Scholar] [CrossRef]
- Johansen, J.S.; Williamson, M.K.; Rice, J.S.; Price, P.A. Identification of proteins secreted by human osteoblastic cells in culture. J. Bone Miner. Res. 1992, 7, 501–512. [Google Scholar]
- Hakala, B.E.; White, C.; Recklies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar]
- Shackelton, L.M.; Mann, D.M.; Millis, A.J.T. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J. Biol. Chem. 1995, 270, 13076–13083. [Google Scholar] [CrossRef]
- Harvey, S.; Weisman, M.; O'Dell, J.; Scott, T.; Krusemeier, M.; Visor, J.; Swindlehurst, C. Chondrex: new marker of joint disease. Clin. Chem. 1998, 44, 509–516. [Google Scholar]
- Bussink, A.P.; Speijer, D.; Aerts, J.M.F.G.; Boot, R.G. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics 2007, 177, 959–970. [Google Scholar] [CrossRef]
- Funkhouser, J.D.; Aronson, N.N. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007, 7, 96. [Google Scholar] [CrossRef]
- Zaheer-ul-Haq; Dala, P.; Aronson, N.N.; Madura, J.D. Family 18 chitolectins: comparison of MGP40 and HUMGP39. Biochem. Biophys. Res. Commun. 2007, 359, 221–226. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Singh, G.; Paramasivam, M.; Saravanan, K.; Jabeen, T.; Sharma, S.; Yadav, S.; Kaur, P.; Kumar, P.; Srinivasan, A.; Singh, T.P. Crystal structure of a novel regulatory 40 kDa mammary gland protein (MGP-40) secreted during involution. J. Biol. Chem. 2003, 278, 14451–14460. [Google Scholar] [CrossRef]
- De Ceuninck, F.; Gaufillier, S.; Bonnaud, A.; Sabatini, M.; Lesur, C.; Pastoureau, P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Biophys. Res. Commun. 2001, 285, 926–931. [Google Scholar] [CrossRef]
- Morrison, B.W.; Leder, P. Neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene 1994, 9, 3417–3426. [Google Scholar]
- Kirkpatrick, R.B.; Matico, R.E.; McNulty, D.E.; Strickler, J.E.; Rosenberg, M. An abundantly secreted glycoprotein from Drosophila melanogaster is related to mammalian secretory proteins produced in rheumatoid tissues and by activated macrophages. Gene 1995, 153, 147–154. [Google Scholar] [CrossRef]
- Kawamura, K.; Shibata, T.; Saget, O.; Peel, D.; Bryant, P.J. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development 1999, 126, 211–219. [Google Scholar]
- Badariotti, F.; Kypriotou, M.; Lelong, C.; Dubos, M.P.; Renard, E.; Galera, P.; Favrel, P. The phylogenetically conserved molluscan chitinase-like protein 1 (Cg-Clp1), homologue of human HC-gp39, stimulates proliferation and regulates synthesis of extracellular matrix components of mammalian chondrocytes. J. Biol. Chem. 2006, 281, 29583–29596. [Google Scholar]
- Renkema, G.H.; Boot, G.R.; Au, F.L.; Donker-Koopman, W.E.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M.F.G. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998, 251, 504–509. [Google Scholar]
- Fusetti, F.; Pijning, T.; Kalk, K.H.; Bos, E.; Dijkstra, B.W. Crystal structure and carbohydrate binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003, 278, 37753–37760. [Google Scholar]
- Houston, D.R.; Recklies, A.D.; Krupa, J.C.; van Aalten, D.M.F. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J. Biol. Chem. 2003, 278, 30206–30212. [Google Scholar]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Johansen, J.S.; Møllgård, K. YKL-40: Common and distinct in cancer and autoimmunity, frequent in early development and abundant in human embryonic stem cells. In International Symposium Honoring Prof. David Naor, Ein Kerem, Jerusalem, Israel, 2–3 September 2007. Abstract 18.
- Ringsholt, M.; Høgdall, E.V.S.; Johansen, J.S.; Price, P.A.; Christensen, L.H. YKL-40 protein expression in normal human tissues - an immunohistochemical study. J. Mol. Histol. 2007, 38, 33–43. [Google Scholar] [CrossRef]
- Johansen, J.S. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodeling, fibrosis and cancer. Dan. Med. Bull. 2006, 53, 172–209. [Google Scholar]
- Johansen, J.S.; Lottenburger, T.; Nielsen, H.J.; Jensen, J.E.B.; Svendsen, M.N.; Kollerup, G.; Christensen, I.B. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2603–2608. [Google Scholar] [CrossRef]
- Johansen, J.S.; Bojesen, S.E.; Mylin, A.K.; Frikke-Schmidt, R.; Price, P.A.; Nordestgaard, B.G. Elevated plasma YKL-40 predicts increased risk of gastrointestinal cancer and decreased survival after any cancer diagnosis in the general population. J. Clin. Oncol. 2009, 27, 572–578. [Google Scholar]
- Fredriksson, S.; Horecka, J.; Brustugun, O.T.; Schlingemann, J.; Koong, A.C.; Tibshirani, R.; Davis, R.W. Multiplexed proximity ligation assays to profile putative plasma biomarkers relevant to pancreatic and ovarian cancer. Clin. Chem. 2008, 54, 582–589. [Google Scholar] [CrossRef]
- Chang, S.T.; Zahn, J.M.; Horecka, J.; Kunz, P.L.; Ford, J.M.; Fisher, G.A.; Le, Q.T.; Chang, D.T.; Ji, H.; Koong, A.C. Identification of a biomarker panel using a multiplex proximity ligation assay improves accuracy of pancreatic cancer diagnosis. J. Transl. Med. 2009, 7, 105. [Google Scholar] [CrossRef]
- Høgdall, E.V.S.; Johansen, J.S.; Kjaer, S.K.; Price, P.A.; Blaakjaer, J.; Høgdall, C.K. Stability of YKL-40 concentration in blood samples. Scand. J. Clin. Lab. Invest. 2000, 60, 247–252. [Google Scholar] [CrossRef]
- Johansen, J.S.; Jensen, H.S.; Price, P.A. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br. J. Rheumatol. 1993, 32, 949–955. [Google Scholar] [CrossRef]
- Johansen, J.S.; Cintin, C.; Jørgensen, M.; Kamby, C.; Price, P.A. Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur. J. Cancer 1995, 31A, 1437–1442. [Google Scholar]
- Roslind, A.; Johansen, J.S. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol. Biol. 2009, 511, 159–184. [Google Scholar] [CrossRef]
- Johansen, J.S.; Jensen, B.V.; Roslind, A.; Nielsen, D.; Price, P.A. Review. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol. Biomarkers Prev. 2006, 15, 194–202. [Google Scholar] [CrossRef]
- Johansen, J.S.; Jensen, B.V.; Roslind, A.; Price, P.A. Review. Is YKL-40 a new therapeutic target in cancer? Expert Opin. Ther. Targets 2007, 11, 219–234. [Google Scholar] [CrossRef]
- Høgdall, E.V.S.; Ringsholt, M.; Høgdall, C.K.; Christensen, I.J.; Johansen, J.S.; Kjaer, S.K.; Blaakaer, J.; Ostenfeld-Møller, L.; Price, P.A.; Christensen, L.H. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer 2009, 9, 8. [Google Scholar] [CrossRef]
- Thöm, I.; Schmid, K.; Burkholder, I.; Johansen, J.S.; Bokemeyer, C.; Schumacher, U.; Laack, E. YKL-40 protein expression in pulmonary adenocarcinoma is not relation to prognosis. ASCO Ann. Meet. Proc. 2010, 28. Abstract e18129. [Google Scholar]
- Bi, J.; Lau, S.H.; Lv, Z.L.; Xie, D.; Li, W.; Lai, Y.R.; Zhong, J.M.; Wu, H.Q.; Su, Q.; He, Y.L.; Zhan, W.H.; Wen, J.M.; Guan, X.Y. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum. Pathol. 2009, 40, 1790–1797. [Google Scholar] [CrossRef]
- Huang, Y.; Prasad, M.; Lemon, W.J.; Hampel, H.; Wright, F.A.; Kornacker, K.; LiVolsi, V.; Frankel, W.; Kloos, R.T.; Eng, C.; Pellegata, N.S.; de la Chapelle, A. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc. Natl. Acad. Sci. USA 2001, 98, 15044–15049. [Google Scholar] [CrossRef]
- Kim, S.H.; Das, K.; Noreen, S.; Coffman, F.; Hameed, M. Prognostic implications of immunohistochemically detected YKL-40 expression in breast cancer. World J. Surg. Oncol. 2007, 5, 17. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Schlatter, L.; Miick, R.; Loy, T.S.; Atasoy, U.; Hewett, J.E.; Sauter, E.R. Increased expression of the inflammatory protein YKL-40 in precancers of the breast. Int. J. Cancer 2007, 121, 1536–1542. [Google Scholar] [CrossRef]
- Roslind, A.; Johansen, J.S.; Junker, N.; Nielsen, D.L.; Dzaferi, H.; Price, P.A.; Balslev, E. YKL-40 expression in benign and malignant lesions of the breast: A methodologic study. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 371–381. [Google Scholar]
- Roslind, A.; Knoop, A.S.; Jensen, M.B.; Johansen, J.S.; Nielsen, D.L.; Price, P.A.; Balslev, E. YKL-40 protein expression is not a prognostic marker in patients with primary breast cancer. Breast Cancer Res. Treat. 2008, 112, 275–285. [Google Scholar] [CrossRef]
- Lau, S.H.; Sham, J.S.T.; Xie, D.; Tzang, C-H.; Tang, D.; Ma, N.; Hu, L.; Wang, Y.; Wen, J.-M.; Xiao, G.; Zhang, W.-M.; Lau, G.K.K.; Yang, M.; Guan, X.-Y. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene 2006, 25, 1242–1250. [Google Scholar] [CrossRef]
- Schunkert, H.; Samani, N.J. Elevated C-reactive protein in atherosclerosis – chicken or egg? Editorial. N. Engl. J. Med. 2008, 359, 1953–1955. [Google Scholar] [CrossRef]
- Nielsen, H.J.; Brünner, N.; Frederiksen, C.; Lomholt, A.F.; King, D.; Jørgensen, L.N.; Olsen, J.; Rahr, H.B.; Thygesen, K.; Hoyer, U.; Laurberg, S.; Christensen, I.J. Danish-Australian Endoscopy Study Group on Colorectal Cancer Detection; Danish Colorectal Cancer Cooperative Group. Plasma tissue inhibitor of metalloproteinases-1 (TIMP-1): a novel biological marker in the detection of primary colorectal cancer. Protocol outlines of the Danish-Australian endoscopy study group on colorectal cancer detection. Scand. J. Gastroenterol. 2008, 43, 242–248. [Google Scholar] [CrossRef]
- Johansen, J.S.; Christensen, I.J.; Price, P.A.; Nielsen, H.J.; The Danish Colorectal Cancer Study Group. . Serum YKL-40 in risk assessment for colorectal cancer. A population based, prospective study of 4987 subjects at risk of colorectal cancer. ASCO Ann. Meet. Proc. 2008, 26, 212, Abstract 4136. [Google Scholar]
- Jensen, B.V.; Johansen, J.S.; Price, P.A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003, 9, 501–512. [Google Scholar]
- Johansen, J.S.; Christensen, I.J.; Riisbro, R.; Greenall, M.; Han, C.; Price, P.A.; Smith, K.; Brünner, N.; Harris, A.L. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res. Treat. 2003, 80, 15–21. [Google Scholar] [CrossRef]
- Coskun, U.; Yamac, D.; Gulbahar, O.; Sancak, B.; Karaman, N.; Ozkan, S. Locally advanced breast carcinoma treated with neoadjuvant chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and MMP-9 correlated with tumor response? Neoplasma 2007, 54, 348–352. [Google Scholar]
- Yamac, D.; Ozturk, B.; Coskun, U.; Tekin, E.; Sancak, B.; Yildiz, R.; Atalay, C. Serum YKL-40 levels as a prognostic factor in patients with locally advanced breast cancer. Adv. Ther. 2008, 25, 801–809. [Google Scholar] [CrossRef]
- Cintin, C.; Johansen, J.S.; Christensen, I.J.; Price, P.A.; Sørensen, S.; Nielsen, H.J. Serum YKL-40 and colorectal cancer. Br. J. Cancer 1999, 79, 1494–1499. [Google Scholar] [CrossRef]
- Cintin, C.; Johansen, J.S.; Christensen, I.J.; Price, P.A.; Sørensen, S.; Nielsen, H.J. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer 2002, 95, 267–274. [Google Scholar] [CrossRef]
- Jensen, B.V.; Spindler, K.-L.G.; Schou, J.V.; Christiansen, I.J.; Høgdall, E.; Nielsen, D.; Johannesen, H.H.; Pfeiffer, P.; Yilmaz, M.; Johansen, J.S. Association of YKL-40 levels in patient with metastatic colorectal cancer treated with third-line cetuximab and irinotecan with short survival. In 2010 Gastrointestinal Cancers Symposium, Orlando, FL, USA, 22–24 January 2010. Abstract 357.
- Diefenbach, C.S.M.; Shah, Z.; Iasonos, A.; Barakat, R.R.; Levine, D.A.; Aghajanian, C.; Sabbatini, P.; Hensley, M.L.; Konner, J.; Tew, W.; Spriggs, D.; Fleisher, M.; Thaler, H.; Dupont, J. Preoperative serum YKL-40 is a marker for detection and prognosis of endometrial cancer. Gynecol. Oncol. 2007, 104, 435–442. [Google Scholar] [CrossRef]
- Thöm, I.; Andritzky, B.; Schuch, G.; Burkholder, I.; Edler, L.; Johansen, J.S.; Bokemeyer, C.; Schumacher, U.; Laack, E. Elevated pre-treatment serum concentration of YKL-40 – an independent prognostic biomarker for poor survival in patients with metastatic non-small cell lung cancer. Cancer 2010. [Google Scholar] [CrossRef]
- Schultz, N.A.; Johnsson, A.; Johansen, J.S.; Christensen, I.J.; Gunnlaugsson, A.; Byström, P.; Nygren, P.; Frödin, J.-E.; Wøjdemann, M.; Glimelius, B.; Berglund, Å. Plasma YKL-40 and IL-6, potential new biomarkers for pancreatic cancer and other upper gastrointestinal adenocarcinomas. BMC Cancer 2010. submitted. [Google Scholar]
- Brasso, K.; Christensen, I.J.; Johansen, J.S.; Teisner, B.; Garnero, P.; Price, P.A.; Iversen, P. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate 2006, 66, 503–513. [Google Scholar] [CrossRef]
- Johansen, J.S.; Brasso, K.; Iversen, P.; Teisner, B.; Garnero, P.; Price, P.A.; Christensen, I.J. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin. Cancer Res. 2007, 13, 3244–3249. [Google Scholar] [CrossRef]
- Kucur, M.; Isman, F.K.; Balci, C.; Onal, B.; Hacibekiroglu, M.; Ozkan, F. Serum YKL-40 levels and chitotriosidase activity as potential biomarkers in primary prostate cancer and benign prostatic hyperplasia. Urol. Oncol. 2008, 26, 47–52. [Google Scholar] [CrossRef]
- Høgdall, E.V.S.; Johansen, J.S.; Kjaer, S.K.; Price, P.A.; Christensen, L.; Blaakaer, J.; Bock, J.E.; Glud, E.; Høgdall, C.K. High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival. Oncol. Rep. 2003, 10, 1535–1538. [Google Scholar]
- Dehn, H.; Høgdall, E.V.S.; Johansen, J.S.; Price, P.A.; Jørgensen, M.; Engelholm, S.A.A.; Høgdall, C.K. Plasma YKL-40, as a prognostic tumor marker in recurrent ovarian cancer. Acta Obstet. Gynecol. Scand. 2003, 82, 287–293. [Google Scholar] [CrossRef]
- Dupont, J.; Tanwar, M.K.; Thaler, H.T.; Fleisher, M.; Kauff, N.; Hensley, M.L.; Sabbatini, P.; Anderson, S.; Aghajanian, C.; Holland, E.C.; Spriggs, D.R. Early detection and prognosis of ovarian cancer using serum YKL-40. J. Clin. Oncol. 2004, 22, 3330–3339. [Google Scholar] [CrossRef]
- Grønlund, B.; Høgdall, E.V.S.; Christensen, I.J.; Johansen, J.S.; Nørgaard-Pedersen, B.; Engelholm, S.A.; Høgdall, C. Pre-treatment prediction of chemoresistance in second-line chemotherapy of ovarian carcinoma: value of serological tumor marker determination (tetranectin, YKL-40, CASA, CA125). Int. J. Biol. Markers 2006, 21, 141–148. [Google Scholar]
- Chudecka-Glaz, A.; Górski, B.; Zielinska, D.; Blogowski, W.; Wojciechowska, I.; Bedner, R.; Rzepka-Górska, I. Serum YKL-40 levels in patients with ovarian cancer and women with BRCA1 gene mutation—comparison to CA 125 antigen. Eur. J. Gynaecol. Oncol. 2009, 30, 668–671. [Google Scholar]
- Mitsuhashi, A.; Matsui, H.; Usui, H.; Nagai, Y.; Tate, S.; Unno, Y.; Hirashiki, K.; Seki, K.; Shozu, M. Serum YKL-40 as a marker for cervical adenocarcinoma. Ann. Oncol. 2009, 20, 71–77. [Google Scholar]
- Johansen, J.S.; Roslind, A.; Palle, C.; Christensen, I.J.; Nielsen, H.J.; Price, P.A.; Nielsen, D.; Mosgaard, B. Serum YKL-40 levels in patients with cervical cancer are elevated compared to patients with cervical intraepithelial neoplasia and healthy controls. ASCO Ann. Meet. Proc. 2006, 24, 267, Abstract 5047. [Google Scholar]
- Roslind, A.; Johansen, J.S.; Christensen, I.J.; Kiss, K.; Balslev, E.; Nielsen, D.L.; Bentzen, J.; Price, P.A.; Andersen, E. High serum levels of YKL-40 in patients with squamous cell carcinoma of the head and neck are associated with short survival. Int. J. Cancer 2008, 122, 857–863. [Google Scholar] [CrossRef]
- Castellano, I.; Mistrangelo, M.; Crudo, V.; Chiusa, L.; Lupo, R.; Ricardi, U.; Morino, M.; Mussa, A.; Cassoni, P. YKL-40 expression in anal carcinoma predicts shorter overall and disease-free survival. Histopathology 2009, 55, 238–240. [Google Scholar] [CrossRef]
- Junker, N.; Johansen, J.S.; Andersen, C.B.; Kristjansen, P.E.G. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer 2005, 48, 223–231. [Google Scholar] [CrossRef]
- Johansen, J.S.; Drivsholm, L.; Price, P.A.; Christensen, I.J. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer 2004, 46, 333–340. [Google Scholar] [CrossRef]
- Geertsen, P.F.; Johansen, J.S.; von der Maase, H.; Jensen, B.V.; Price, P.A. High pretreatment serum level of YKL-40 is related to short survival in patients with advanced renal cell carcinoma treated with high-dose continuous intravenous infusion of interleukin-2. ASCO 2004. Ann. Meet. Proc. 2003, 22, 399, Abstract 1603. [Google Scholar]
- Schmidt, H.; Johansen, J.S.; Gehl, J.; Geertsen, P.F.; Fode, K.; von der Maase, H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer 2006, 106, 1130–1139. [Google Scholar] [CrossRef]
- Schmidt, H.; Johansen, J.S.; Sjoegren, P.; Christensen, I.J.; Sørensen, B.S.; Fode, K.; Larsen, J.; von der Maase, H. Serum YKL-40 predicts relapse-free and overall survival in patients with American Joint Committee on Cancer stage I and II melanoma. J. Clin. Oncol. 2006, 24, 798–804. [Google Scholar] [CrossRef]
- Krogh, M.; Christensen, I.J.; Bouwhuis, M.; Johansen, J.S.; Schmidt, H.; Hansson, J.; Aamdal, S.; Testori, A.; Eggermont, A.M.; Bastholt, L. Prognostic value of serum YKL-40 in stage IIB-III melanoma patients receiving adjuvant interferon therapy. ASCO Ann. Meet. Proc. 2010. Abstract 8587. [Google Scholar]
- Junker, N.; Johansen, J.S.; Hansen, L.T.; Lund, E.L.; Kristjansen, P.E.G. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005, 96, 183–190. [Google Scholar] [CrossRef]
- Saidi, A.; Javerzat, S.; Bellahcene, A.; De Vos, J.; Bello, L.; Castronovo, V.; Deprez, M.; Loiseau, H.; Bikfalvi, A.; Hagedorn, M. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. Int. J. Cancer 2008, 122, 2187–2198. [Google Scholar]
- Krona, A.; Aman, P.; Orndal, C.; Josefsson, A. Oncostatin M-induced genes in human astrocytomas. Int. J. Oncol. 2007, 31, 1457–1463. [Google Scholar]
- Lal, A.; Lash, A.E.; Altschul, S.F.; Velculescu, V.; Zhang, L.; McLendon, R.E.; Marra, M.A.; Prange, C.; Morin, P.J.; Polyak, K.; Papadopoulos, N.; Vogelstein, B.; Kinzler, K.W.; Strausberg, R.L.; Riggins, G.J. A public database for gene expression in human cancers. Cancer Res. 1999, 59, 5403–5407. [Google Scholar]
- Markert, J.M.; Fuller, C.M.; Gillespie, G.Y.; Bubien, J.K.; McLean, L.A.; Hong, R.L.; Lee, K.; Gullans, S.R.; Mapstone, T.B.; Benos, D.J. Differential gene expression profiling in human brain tumors. Physiol. Genomics 2001, 5, 21–33. [Google Scholar]
- Tanwar, M.K.; Gilbert, M.R.; Holland, E.C. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002, 62, 4364–4368. [Google Scholar]
- Shostak, K.; Labunskyy, V.; Dmitrenko, V.; Malisheva, T.; Shamayev, M.; Rozumenko, V.; Zozulya, Y.; Zehetner, G.; Kavsan, V. HC gp-39 gene is upregulated in glioblastoma. Cancer Lett. 2003, 198, 203–210. [Google Scholar] [CrossRef]
- Colin, C.; Baeza, N.; Bartoli, C.; Fina, F.; Eudes, N.; Nanni, I.; Martin, P.-M.; Ouafik, L.; Figarella-Branger, D. Identification of genes differentially expressed in glioblastoma versus pilocytic astrocytoma using Suppression Subtractive Hybridization. Oncogene 2006, 25, 2818–2829. [Google Scholar] [CrossRef]
- Nigro, J.M.; Misra, A.; Zhang, L.; Smirnov, I.; Colman, H.; Griffin, C.; Ozburn, N.; Chen, M.; Pan, E.; Koul, D.; Yung, W.K.A.; Feuerstein, B.G.; Aldape, K.D. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005, 65, 1678–1686. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; Williams, P.M.; Modrusan, Z.; Feuerstein, B.G.; Aldape, K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Kroes, R.A.; Dawson, G.; Moskal, J.R. Focused microarray analysis of glyco-gene expression in human glioblastomas. J. Neurochem. 2007, 103, 14–24. [Google Scholar] [CrossRef]
- Ducray, F.; Idbaih, A.; de Reynies, A.; Bieche, I.; Thillet, J.; Mokhtari, K.; Lair, S.; Marie, Y.; Paris, S.; Vidaud, M.; Hoang-Xuan, K.; Delattre, O.; Delattre, J.-Y.; Sanson, M. Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol. Cancer 2008, 7, 41. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pelloski, C.E.; Zhang, Y.; Kim, S.H.; delaCruz, C.; Rehli, M.; Aldape, K.B. Selective repression of YKL-40 by NF-kappaB in glioma cell lines involves recruitment of histone deacetylase-1 and -2. FEBS Lett. 2008, 582, 3193–3200. [Google Scholar] [CrossRef]
- Pelloski, C.E.; Mahajan, A.; Maor, M.; Chang, E.L.; Woo, S.; Gilbert, M.; Colman, H.; Yang, H.; Ledoux, A.; Blair, H.; Passe, S.; Jenkins, R.B.; Aldape, K.D. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin. Cancer Res. 2005, 11, 3326–3334. [Google Scholar] [CrossRef]
- Nutt, C.L.; Betensky, R.A.; Brower, M.A.; Batchelor, T.T.; Louis, D.N.; Stemmer-Rachamimow, A.O. YKL-40 is a differential diagnostic marker for histologic subtypes of high-grade gliomas. Clin. Cancer Res. 2005, 11, 2258–2264. [Google Scholar] [CrossRef]
- Pelloski, C.E.; Ballman, K.V.; Furth, A.F.; Zhang, L.; Lin, E.; Sulman, E.P.; Bhat, K.; McDonald, J.M.; Yung, W.K.A.; Colman, H.; Woo, S.Y.; Heimberger, A.B.; Suki, D.; Prados, M.D.; Chang, S.M.; Barker, F.G., II; Buckner, J.C.; James, D.; Aldape, K. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 2007, 25, 2288–2294. [Google Scholar] [CrossRef]
- Rousseau, A.; Nutt, C.L.; Betensky, R.A.; Iafrate, J.; Han, M.; Ligon, K.L.; Rowitch, D.H.; Louis, D.N. Expression of oligodendroglial and astrocytic lineage markers in diffuse gliomas: use of YKL-40, ApoE, ASCL1, and NKX2-2. J. Neuropathol. Exp. Neurol. 2006, 65, 1149–1156. [Google Scholar] [CrossRef]
- Horbinski, C.; Wang, G.; Wiley, C.A. YKL-40 is directly produced by tumor cells and is inversely linked to EGFR in glioblastomas. Int. J. Clin. Exp. Pathol. 2010, 3, 226–237. [Google Scholar]
- Hormigo, A.; Gu, B.; Karimi, S.; Riedel, E.; Panageas, K.S.; Edgar, M.A.; Tanwar, M.K.; Rao, J.S.; Fleisher, M.; DeAngelis, L.M.; Holland, E.C. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin. Cancer Res. 2006, 12, 5698–5704. [Google Scholar] [CrossRef]
- Antonelli, M.; Buttarelli, F.R.; Arcella, A.; Nobusawa, S.; Donofrio, V.; Oghaki, H.; Giangaspero, F. Prognostic significance of histological grading, p53 status, YKL-40 expression, and IDH1 mutations in pediatric high-grade gliomas. J. Neurooncol. 2010. [Google Scholar] [CrossRef]
- Mylin, A.K.; Rasmussen, T.; Johansen, J.S.; Knudsen, L.M.; Nørgaard, P.H.; Lenhoff, S.; Dahl, I.M.S.; Johnsen, H.E. Serum YKL-40 concentrations in newly diagnosed multiple myeloma patients and YKL-40 expression in malignant plasma cells. Eur. J. Hematol. 2006, 77, 416–424. [Google Scholar] [CrossRef]
- Kirkpatrick, R.B.; Emery, J.G.; Connor, J.R.; Dodds, R.; Lysko, P.G.; Rosenberg, M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 1997, 237, 46–54. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Bijlsma, S.; De Groene, E.M.; Witkamp, R.F.; van der Greef, F.; Rodenburg, R.J.T. A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkes for macrophage maturation in the U937 cell line. Proteomics 2004, 4, 1014–1028. [Google Scholar] [CrossRef]
- Bergmann, O.J.; Johansen, J.S.; Klausen, T.W.; Mylin, A.K.; Kristensen, J.S.; Kjeldsen, E.; Johnsen, H.E. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin. Cancer Res. 2005, 11, 8644–8652. [Google Scholar] [CrossRef]
- Mylin, A.K.; Abildgaard, N.; Johansen, J.S.; Andersen, N.F.; Heickendorff, L.; Standal, T.; Gimsing, P.; Knudsen, L.M. High serum YKL-40 concentration is associated with severe bone disease in newly diagnosed multiple myeloma patients. Eur. J. Haematol. 2008, 80, 310–317. [Google Scholar] [CrossRef]
- Mylin, A.K.; Andersen, N.F.; Johansen, J.S.; Abildgaard, N.; Heickendorff, L.; Standal, T.; Gimsing, P.; Knudsen, L.M. Serum YKL-40 and bone marrow angiogenesis in multiple myeloma. Int. J. Cancer 2009, 124, 1492–1494. [Google Scholar] [CrossRef]
- Biggar, R,J.; Johansen, J.S.; Smedby, K.E.; Rostgaard, K.; Chang, E.T.; Adami, H-O.; Glimelius, B.; Molin, D.; Hamilton-Dutoit, S.; Melbye, M.; Hjalgrim, H. Serum YKL-40 and IL-6 levels in Hodgkin lymphoma. Clin. Cancer Res. 2008, 14, 6974–6979. [Google Scholar] [CrossRef]
- Lee, C.G.; Elias, J.A. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol. Res. 2010, 2, 20–27. [Google Scholar] [CrossRef]
- Johansen, J.S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K.; Bruunsgaard, H. High serum YKL-40 level in a cohort of 80-years old is associated with increased risk of all-cause mortality. Clin. Exp. Immunol. 2008, 151, 260–266. [Google Scholar]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Kushner, I.; Rzewnicki, D.; Samols, D. What does minor elevation of C-reactive protein signify? Am. J. Med. 2006, 166, e17–28. [Google Scholar]
- Rathcke, C.N.; Raymond, I.; Kistorp, C.; Hildebrandt, P.; Faber, J.; Vestergaard, H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int. J. Cardiol. 2009. [Google Scholar] [CrossRef]
- Volck, B.; Price, P.A.; Johansen, J.S.; Sørensen, O.; Benfield, T.; Nielsen, H.J.; Calafat, J.; Borregaard, N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Phys. 1998, 110, 351–360. [Google Scholar]
- Nordenbæk, C.; Johansen, J.S.; Junker, P.; Borregaard, N.; Sørensen, O.; Price, P.A. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J. Infect. Dis. 1999, 180, 1722–1726. [Google Scholar] [CrossRef]
- Kronborg, G.; Østergaard, C.; Weis, N.; Nielsen, H.; Obel, N.; Pedersen, S.S.; Price, P.A.; Johansen, J.S. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated with the outcome of the disease. Scand. J. Infect. Dis. 2002, 34, 323–326. [Google Scholar] [CrossRef]
- Hattori, N.; Oda, S.; Sadahiro, T.; Nakamura, M.; Abe, R.; Shinozaki, K.; Nomura, F.; Tomonaga, T.; Matsushita, K.; Kodera, Y.; Sogawa, K.; Satoh, M.; Hirasawa, H. YKL-40 identified by proteomic analysis as a biomarker of sepsis. Shock 2009, 32, 393–400. [Google Scholar] [CrossRef]
- Johansen, J.S.; Krabbe, K.; Møller, K.; Pedersen, B.K. Circulating YKL-40 levels during human endotoxaemia. Clin. Exp. Immunol. 2005, 140, 343–348. [Google Scholar] [CrossRef]
- Østergaard, C.; Johansen, J.S.; Benfield, T.; Price, P.A.; Lundgren, J.D. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab. Immun. 2002, 9, 598–604. [Google Scholar]
- Boot, R.G.; van Achterberg, T.A.E.; van Aken, B.E.; Renkema, G.H.; Jacobs, M.J.H.M.; Aerts, J.M.F.G.; de Vries, C.J.M. Strong induction of members of the chitinase family of proteins in atherosclerosis. Chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 687–694. [Google Scholar] [CrossRef]
- Millis, A.J.T.; Hoyle, M.; Reich, E.; Mann, D.M. Isolation and characterization of a Mr = 38,000 protein from differentiating smooth muscle cells. J. Biol. Chem. 1985, 260, 3754–3761. [Google Scholar]
- Millis, A.J.T.; Hoyle, M.; Kent, L. In vitro expression of a 38,000 dalton heparin-binding glycoprotein by morphologically differentiated smooth muscle cells. J. Cell Physiol. 1986, 127, 366–372. [Google Scholar] [CrossRef]
- Malinda, K.M.; Ponce, L.; Kleinman, H.K.; Shackelton, L.M.; Millis, A.J.T. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp. Cell Res. 1999, 250, 168–173. [Google Scholar] [CrossRef]
- Nishikawa, K.C.; Millis, A.J.T. Gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp. Cell Res. 2003, 287, 79–87. [Google Scholar] [CrossRef]
- Johansen, J.S.; Baslund, B.; Garbarsch, C.; Hansen, M.; Stoltenberg, M.; Lorenzen, I.; Price, P.A. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999, 42, 2624–2630. [Google Scholar] [CrossRef]
- Nøjgaard, C.; Høst, N.B.; Christensen, I.J.; Poulsen, S.H.; Egstrup, K.; Price, P.A.; Johansen, J.S. Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coron. Artery Dis. 2008, 19, 257–263. [Google Scholar] [CrossRef]
- Wang, Y.; Ripa, R.S.; Johansen, J.S.; Gabrielsen, A.; Steinbrüchel, D.A.; Friis, T.; Bindslev, L.; Haack-Sørensen, M.; Jørgensen, E.; Kastrup, J. YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand. Card. J. 2008, 42, 295–302. [Google Scholar] [CrossRef]
- Kucur, M.; Isman, F.K.; Karadag, B.; Vural, V.A.; Tavsanoglu, S. Serum YKL-40 levels in patients with coronary artery disease. Coron. Artery Dis. 2007, 18, 391–396. [Google Scholar] [CrossRef]
- Zheng, J.L.; Lu, L.; Hu, J.; Zhang, R.Y.; Zhang, Q.; Chen, Q.J.; Shen, W.F. Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis 2010, 210, 590–595. [Google Scholar]
- Henningsen, K.M.; Therkelsen, S.K.; Johansen, J.S.; Bruunsgaard, H.; Svendsen, J.H. Plasma YKL-40, a new biomarker for artrial fibrillation? Europace 2009, 11, 1032–1036. [Google Scholar] [CrossRef]
- Henningsen, K.M.; Nilsson, B.; Johansen, J.S.; Chen, X.; Pehrson, S.; Svendsen, J.H. Plasma YKL-40 is elevated in patients with recurrent atrial fibrillation after catheter ablation. Inflamm. Res. 2010, 59, 463–469. [Google Scholar] [CrossRef]
- Kastrup, J.; Johansen, J.S.; Winkel, P.; Hansen, J.F.; Hildebrandt, P.; Jensen, G.B.; Jespersen, C.M.; Kjøller, E.; Kolmos, H.J.; Lind, I.M.; Nielsen, H.; Gluud, C.; The CLARICOR Trial Group. High serum concentration of YKL-40 in patients with stable coronary artery disease is associated with increased risk of myocardial infarction, cardiovascular death and all-cause mortality. Eur. Heart J. 2009, 30, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- Rathcke, C.N.; Kistorp, C.; Raymond, I.; Hildebrandt, P.; Gustafsson, F.; Lip, G.Y.; Faber, J.; Vestergaard, H. Plasma YKL-40 levels are elevated in patients with chronic heart failure. Scand. Cardiovasc. J. 2009. Dec 4 Epub ahead of print. [Google Scholar]
- Hedegaard, A.; Ripa, R.S.; Johansen, J.S.; Jørgensen, E.; Kastrup, J. Plasma YKL-40 and recovery of left ventricular function after acute myocardial infarction. Scand. J. Clin. Lab. Invest. 2010, 70, 80–86. [Google Scholar] [CrossRef]
- Rathcke, C.N.; Johansen, J.S.; Vestergaard, H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm. Res. 2006, 55, 53–59. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Erikstrup, C.; Johansen, J.S.; Fischer, C.P.; Plomgaard, P.; Krogh-Madsen, R.; Taudorf, S.; Mortensen, O.H.; Petersen, A.M.W.; Lindegaard, B.; Pedersen, B.K. Plasma YKL-40: a BMI-independent marker of type 2 diabetes. Diabetes 2008, 57, 3078–3082. [Google Scholar] [CrossRef]
- Rathcke, C.N.; Persson, F.; Tarnow, L.; Rossing, P.; Vestergaard, H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care 2009, 32, 323–328. [Google Scholar]
- Rathcke, C.N.; Vestergaard, H. YKL-40—an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc. Diabetol. 2009, 8, 61. [Google Scholar] [CrossRef]
- Volck, B.; Johansen, J.S.; Stoltenberg, M.; Garbarsch, C.; Price, P.A.; Østergaard, M.; Østergaard, K.; Løvgreen-Nielsen, P.; Sonne-Holm, S.; Lorenzen, I. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthr. Cartil. 2001, 9, 203–214. [Google Scholar] [CrossRef]
- Johansen, J.S.; Olee, T.; Price, P.A.; Hashimoto, S.; Ochs, R.L.; Lotz, M. Regulation of YKL-40 production by human articular chondrocytes. Arthritis Rheum. 2001, 44, 826–837. [Google Scholar] [CrossRef]
- Nyirkos, P.; Golds, E.E. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem. J. 1990, 268, 265–268. [Google Scholar]
- Dasuri, K.; Antonovici, M.; Chen, K.; Wong, K.; Standing, K.; Ens, W.; El-Gabalawy, H.; Wilkins, J.A. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthritis Res. Ther. 2004, 6, R161–168. [Google Scholar] [CrossRef] [Green Version]
- Baeten, D.; Boots, A.M.H.; Steenbakkers, P.G.A.; Elewaut, D.; Bos, E.; Verheijden, G.F.M.; Verbruggen, G.; Miltenburg, A.M.M.; Rijnders, A.W.M.; Veys, E.M.; de Keyser, F. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium.Correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1233–1243. [Google Scholar] [CrossRef]
- Belge, K.U.; Dayyani, F.; Horelt, A.; Siedlar, M.; Frankenberger, M.; Frankenberger, B.; Espevik, T.; Ziegler-Heitbrock, L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 2002, 168, 3536–3542. [Google Scholar]
- Johansen, J.S.; Stoltenberg, M.; Hansen, M.; Florescu, A.; Hørslev-Petersen, K.; Lorenzen, I.; Price, P.A. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology 1999, 38, 618–626. [Google Scholar] [CrossRef]
- Johansen, J.S.; Kirwan, J.R.; Price, P.A.; Sharif, M. Serum YKL-40 concentrations in patients with early rheumatoid arthritis: relation to joint destruction. Scand. J. Rheumatol. 2001, 30, 297–304. [Google Scholar] [CrossRef]
- Harvey, S.; Whaley, J.; Eberhardt, K. The relationship between serum levels of YKL-40 and disease progression in patients with early rheumatoid arthritis. Scand. J. Rheumatol. 2000, 29, 391–393. [Google Scholar] [CrossRef]
- Vos, K.; Steenbakkers, P.; Miltenburg, A.M.M.; Bos, E.; van den Heuvel, M.V.; van Hogezand, R.A.; de Vries, R.R.P.; Breedveld, F.C.; Boots, A.M.H. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 2000, 59, 544–548. [Google Scholar] [CrossRef]
- Combe, B.; Dougados, M.; Goupille, P.; Cantagrel, A.; Eliaou, J.F.; Sibilia, J.; Meyer, O.; Sany, J.; Daures, J.-P.; Dubois, A. Prognostic factors for radiographic damage in early rheumatoid arthritis: a multiparameter prospective study. Arthritis Rheum. 2001, 44, 1736–1743. [Google Scholar] [CrossRef]
- Peltomaa, R.; Paimela, L.; Harvey, S.; Helve, T.; Leirisalo-Repo, M. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: a new marker for disease activity. Rheumatol. Int. 2001, 20, 192–196. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tsurumoto, T. Serum YKL-40 levels in rheumatoid arthritis: correlations between clinical and laboratory parameters. Clin. Exp. Rheumatol. 2001, 19, 655–660. [Google Scholar]
- Knudsen, L.S.; Østergaard, M.; Baslund, B.; Narvestad, E.; Petersen, J.; Nielsen, H.J.; Ejbjerg, B.J.; Szkudlarek, M.; Johansen, J.S. Plasma IL-6, plasma VEGF and serum YKL-40: relationship with disease activity and radiographic progression in rheumatoid arthritis patients treated with infliximab and methotrexate. Scand. J. Rheumatol. 2006, 35, 489–491. [Google Scholar] [CrossRef]
- Knudsen, L.S.; Klarlund, M.; Skjødt, H.; Jensen, T.; Østergaard, M.; Jensen, K.E.; Hansen, M.S.; Hetland, M.L.; Nielsen, H.J.; Johansen, J.S. Biomarkers of inflammation in patients with unclassified polyarthritis and early rheumatoid arthritis. Relationship to disease activity and radiographic outcome. J. Rheumatol. 2008, 35, 1277–1287. [Google Scholar]
- Sharif, M.; Granell, R.; Johansen, J.S.; Clarke, S.; Elson, C.; Kirwan, J.R. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology 2006, 5, 522–526. [Google Scholar]
- Chupp, G.L.; Lee, C.G.; Jarjour, N.; Shim, Y.M.; Holm, C.T.; He, S.; Dziura, J.D.; Reed, J.; Coyle, A.J.; Kiener, P.; Cullen, M.; Grandsaigne, M.; Dombret, M.-C.; Aubier, M.; Pretolani, M.; Elias, J.A. A chitinase–like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007, 357, 2016–2027. [Google Scholar] [CrossRef]
- Letuve, S.; Kozhich, A.; Arouche, N.; Grandsaigne, M.; Reed, J.; Dombret, M.-C.; Kiener, P.A.; Aubier, M.; Coyle, A.J.; Pretolani, M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008, 181, 5167–5173. [Google Scholar]
- Johansen, J.S.; Milman, N.; Hansen, M.; Garbarsch, C.; Price, P.A.; Graudal, N. Increased serum YKL-40 in patients with pulmonary sarcoidosis. A potential marker of disease activity? Respir. Med 2005, 99, 396–402. [Google Scholar] [CrossRef]
- Kuepper, M.; Bratke, K.; Virchow, J.C. Chitinase-like protein and asthma. N. Engl. J. Med. 2008, 358, 1073–1075. [Google Scholar] [CrossRef]
- Montagna, G.L.; D’Angelo, S.; Valentini, G. Cross-sectional evaluation of YKL-40 serum concentrations in patients with systemic sclerosis. Relationship with clinical and serological aspects of disease. J. Rheumatol. 2003, 30, 2147–2151. [Google Scholar]
- Nordenbæk, C.: Johansen, J.S.; Halberg, P.; Wiik, A.; Garbarsch, C.; Ullman, S.; Price, P.A.; Jacobsen, S. High serum levels of YKL-40 in patients with systemic sclerosis are associated with pulmonary involvement. Scand. J. Rheumatol. 2005, 34, 293–297. [Google Scholar] [CrossRef]
- Ober, C.; Chupp, G.L. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 401–408. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Petinaki, E.; Dimoulios, P.; Vardas, E.; Roussomoustakaki, M.; Maniatis, A.N.; Kouroumalis, E.A. Increased serum levels of YKL-40 in patients with inflammatory bowel disease. Int. J. Colorectal Dis. 2003, 18, 254–259. [Google Scholar]
- Punzi, L.; Podswiadek, M.; D’Inca, R.; Zaninotto, M.; Bernardi, D.; Plebani, M.; Sturniolo, G.C. Serum human cartilage glycoprotein 39 as a marker of arthritis associated with inflammatory bowel disease. Ann. Rheum. Dis. 2003, 62, 1224–1226. [Google Scholar] [CrossRef]
- Vind, I.; Johansen, J.S.; Price, P.A.; Munkholm, P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2003, 38, 599–605. [Google Scholar] [CrossRef]
- Erzin, Y.; Uzun, H.; Karatas, A.; Celik, A.F. Serum YKL-40 as a marker of disease activity and stricture formation in patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2008, 23, e357–362. [Google Scholar] [CrossRef]
- Johansen, J.S.; Møller, S.; Price, P.A.; Bendtsen, F.; Junge, J.; Garbarsch, C.; Henriksen, J.H. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand. Scand. J. Gastroenterol. 1997, 32, 582–590. [Google Scholar] [CrossRef]
- Johansen, J.S.; Christoffersen, P.; Møller, S.; Price, P.A.; Henriksen, J.H.; Garbarsch, C.; Bendtsen, F. Serum YKL-40 is increased in patients with hepatic fibrosis. J. Hepatol. 2000, 32, 911–920. [Google Scholar] [CrossRef]
- Berres, M.L.; Papen, S.; Pauels, K.; Schmitz, P.; Zaldivar, M.M.; Hellerbrand, C.; Mueller, T.; Berg, T.; Weiskirchen, R.; Trautwein, C.; Wasmuth, H.E. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J. Hepatol. 2009, 50, 370–376. [Google Scholar] [CrossRef]
- Nøjgaard, C.; Johansen, J.S.; Christensen, E.; Skovgaard, L.T.; Price, P.A.; Becker, U.; The EMALD Group. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J. Hepatol. 2003, 39, 179–186. [Google Scholar] [CrossRef]
- Tran, A.; Benzaken, S.; Saint-Paul, M.C.; Guzman-Granier, E.; Hastier, P.; Pradier, C.; Barjoan, E.M.; Demuth, N.; Longo, F.; Rampal, P. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur. J. Gastroenterol Hepatol. 2000, 12, 989–993. [Google Scholar] [CrossRef]
- Nøjgaard, C.; Johansen, J.S.; Krarup, H.B.; Holten-Andersen, M.; Møller, A.; Bendtsen, F.; Danish Viral Hepatitis Study Group. Effect of antiviral therapy on markers of fibrogenesis in patients with chronic hepatitis C. Scand. J. Gastroenterol. 2003, 38, 659–665. [Google Scholar] [CrossRef]
- Nunes, D.; Fleming, C.; Offner, G.; O’Brien, M.; Tumilty, S.; Fix, O.; Heeren, T.; Koziel, M.; Graham, C.; Craven, D.E.; Stuver, S.; Horsburgh, C.R., Jr. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J. Acquir. Immune. Defic. Syndr. 2005, 40, 538–544. [Google Scholar] [CrossRef]
- Zheng, M.; Cai, W.M.; Zhao, J.K.; Zhu, S.M.; Liu, R.H. Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop. 2005, 96, 148–152. [Google Scholar] [CrossRef]
- Kamal, S.M.; Turner, B.; He, Q.; Rasenack, J.; Bianchi, L.; Tawil, A.A.; Nooman, A.; Massoud, M.; Koziel, M.J.; Afdhal, N.H. Progression of fibrosis in hepatitis C with and without schistosomiasis: correlation with serum markers of fibrosis. Hepatology 2006, 43, 771–779. [Google Scholar] [CrossRef]
- Alexander, J.; Tung, B.Y.; Croghan, A.; Kowdley, K.V. Effect of iron depletion on serum markers of fibrogenesis, oxidative stress and serum liver enzymes in chronic hepatitis C: results of a pilot study. Liver Int. 2007, 27, 268–273. [Google Scholar] [CrossRef]
- Esmat, G.; Metwally, M.; Zalata, K.R.; Gadalla, S.; Abdel-Hamid, M.; Abouzied, A.; Shaheen, A.-A.; El-Raziky, M.; Khatab, H.; El-Kafrawy, S.; Mikhail, N.; Magder, L.S.; Afdhal, N.H.; Strickland, G.T. Evaluation of serum biomarkers of fibrosis and injury in Egyptian patients with chronic hepatitis C. J. Hepatol. 2007, 46, 620–627. [Google Scholar] [CrossRef]
- Fontana, R.J.; Goodman, Z.D.; Dienstag, J.L.; Bonkovsky, H.L.; Naishadham, D.; Sterling, R.K.; Su, G.L.; Ghosh, M.; Wright, E.C.; the HALT-C Trial Group. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology 2008, 47, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Fontana, R.J.; Bonkovsky, H.L.; Naishadham, D.; Dienstag, J.L.; Sterling, R.K.; Lok, A.S.; Su, G.L.; Halt-C Trial Group. Serum fibrosis marker levels decrease after successful antiviral treatment in chronic hepatitis C patients with advanced fibrosis. Clin. Gastroenterol. Hepatol. 2009, 7, 219–226. [Google Scholar] [CrossRef]
- Lebensztejn, D.M.; Skiba, E.; Werpachowska, I.; Sobaniec-Lotowska, M.E.; Kaczmarksi, M. Serum level of YKL-40 does not predict advanced liver fibrosis in children with chronic hepatitis B. Adv. Med. Sci. 2007, 52, 120–124. [Google Scholar]
- Pungpapong, S.; Nunes, D.P.; Krishna, M.; Nakhieh, R.; Chambers, K.; Ghabril, M.; Dickson, R.C.; Hughes, C.B.; Steers, J.; Nguyen, J.H.; Keaveny, A.P. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transpl. 2008, 14, 1294–1302. [Google Scholar] [CrossRef]
- Mehta, P.; Ploutz-Snyder, R.; Nandi, J.; Rawlins, S.R.; Sanderson, S.O.; Levine, R.A. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am. J. Gastroenterol 2008, 103, 928–936. [Google Scholar] [CrossRef]
- Schiavon, L.L.; Narciso-Schiavon, J.L.; Carvalho-Filho, R.J.; Sampaio, J.P.; Medina-Pestana, J.O.; Lanzoni, V.P.; Silva, A.E.; Ferraz, M.L. Serum levels of YKL-40 and hyaluronic acid as non-invasive markers of liver fibrosis in haemodialysis patients with chronic hepatitis C virus infection. J. Viral. Hepat. 2008, 15, 666–674. [Google Scholar] [CrossRef]
- Lee, K.G.; Seo, Y.S.; An, H.; Um, S.H.; Jung, E.S.; Keum, B.; Yim, H.J.; Jeen, Y.T.; Chun, H.J.; Kim, C.D.; Ryu, H.S. Usefulness of non-invasive markers for predicting liver cirrhosis in patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 2010, 25, 94–100. [Google Scholar] [CrossRef]
- Schiavon, L.L.; Carvalho-Filho, R.J.; Narciso-Schiavon, J.L.; Medina-Pestana, J.O.; Lanzoni, V.P.; Ferraz, M.L.; Silva, A.E. YKL-40 and hyaluronic acid (HA) as noninvasive markers of liver fibrosis in kidney transplant patients with HCV chronic infection. Scand. J. Gastroenterol. 2010, 45, 615–622. [Google Scholar] [CrossRef]
- Recklies, A.D.; White, C.; Ling, H. The chitinase 3-like protein human cartilage 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase-and protein kinase B-mediated signalling pathways. Biochem. J. 2002, 365, 119–126. [Google Scholar] [CrossRef]
- Recklies, A.D.; Ling, H.; White, C.; Bernier, S.M. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J. Biol. Chem. 2005, 280, 41213–41221. [Google Scholar] [CrossRef]
- Ling, H.; Recklies, A.D. The chitinase 3-like protein human cartilage glycoproein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumor necrosis factor-alpha. Biochem. J. 2004, 380, 651–659. [Google Scholar] [CrossRef]
- Biosselier, B.; Marie, Y.; El Hallani, S.; Kaloshi, G.; Iershov, A.; Kavsan, V.; Psimaras, D.; Thillet, J.; Hoang-Xuan, K.; Delattre, J.Y.; Sanson, M. No association of (−131C→G) variant of CHI3L1 gene with risk of glioblastoma and prognosis. J. Neurooncol. 2009, 94, 169–172. [Google Scholar] [CrossRef]
- Ober, C.; Tan, Z.; Sun, Y.; Possick, J.D.; Pan, L.; Nicolae, R.; Radford, S.; Parry, R.R.; Heinzmann, A.; Deichmann, K.A.; Lester, L.A.; Gern, J.E.; Lemanske, R.F., Jr.; Nicolae, D.L.; Elias, J.A.; Chupp, G.L. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N. Engl. J. Med. 2008, 358, 1682–1691. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, R.; Gao, B.; Shi, Y.; Zhou, J.; Guo, S.; Zhang, J.; Wang, Y.; Tang, W.; Meng, J.; Li, S.; Wang, H.; Ma, G.; Lin, C.; Xiao, Y.; Feng, G.; Lin, Z.; Zhu, S.; Xing, Y.; Sang, H.; St. Clair, D.; He, L. Functional variants in the promoter region of Chitinase 3-Like 1 (CHI3L1) and susceptibility to schizophrenia. Am. J. Hum. Genet. 2007, 80, 12–18. [Google Scholar] [CrossRef]
- Yang, M.S.; Morris, D.W.; Donohoe, G.; Kenny, E., O’Dushalaine; Schwaiger, S.; Nangle, J.M.; Clarke, S.; Scully, P.; Quinn, J.; Meagher, D.; Baldwin, P.; Crumlish, N.; O’Callaghan, E.; Waddington, J.L.; Gill, M.; Corvin, A. Chitinase-3-like 1 (CHI3L1) gene and schizophrenia: genetic association and a potential functional mechanism. Biol. Psychiatry 2008, 64, 98–103. [Google Scholar] [CrossRef]
- Ohi, K.; Hashimoto, R.; Yasuda, Y.; Yoshida, T.; Takahashi, H.; Iike, N.; Iwase, M.; Kamino, K.; Ishii, R.; Kazui, H.; Fukumoto, M.; Takamura, H.; Yamamori, H.; Azechi, M.; Ikezawa, K.; Tanimukai, H.; Tagami, S.; Morihara, T.; Okochi, M.; Yamada, K.; Numata, S.; Ikeda, M.; Tanaka, T.; Kudo, T.; Ueno, S.; Yoshikawa, T.; Ohmori, T.; Iwata, N.; Ozaki, N.; Takeda, M. The chitinase 3-like 1 gene and schizophrenia: evidence from a multi-center case-control study and meta-analysis. Schizophr. Res. 2010, 116, 126–132. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Antal, P.; Gal, J.; Hullam, G.; Semsei, A.F.; Nagy, G.; Falus, A.; Buzas, E.I. Lack of evidence for association of two functional SNPs of CHI3L1 gene (HC-gp39) with rheumatoid arthritis. Rheumatol. Int. 2010. [Google Scholar] [CrossRef]
- Kruit, A.; Grutters, J.C.; Ruven, H.J.T.; van Moorsel, C.C.; van den Bosch, J.M. A CHI3L1 gene polymorphism is associated with serum levels of YKL-40, a novel sarcoidosis marker. Respir. Med. 2007, 101, 1563–1571. [Google Scholar] [CrossRef]
- Rathcke, C.N.; Holmkvist, J.; Jørgensen, T.; Borch-Johnsen, K.; Hansen, T.; Pedersen, O.B.; Vestergaard, H. Variation in CHI3LI in relation to type 2 diabetes and related quantitative traits. PloS One 2009, 4, e5469. [Google Scholar] [CrossRef]
- Rathcke, C.N.; Holmkvist, J.; Husmoen, L.L.; Hansen, T.; Pedersen, O.; Vestergaard, H.; Linneberg, A. Association of polymorphisms of the CHI3L1 gene with asthma and atopy: a populations-based study of 6514 Danish adults. PloS One 2009, 4, e6106. [Google Scholar] [CrossRef]
- Krause, S.W.; Rehli, M.; Kreutz, M.; Schwarzfisher, L.; Paulauski, J.D.; Andreesen, R. Differential screening identifies genetic markers of monocyte to macrophage maturation. J. Leukocyte Biol. 1996, 60, 540–545. [Google Scholar]
- Hashimoto, S.; Suzuki, T.; Dong, H.Y.; Yamazaki, N.; Matsushima, K. Serial analysis of gene expression in human monocytes and macrophages. Blood 1999, 94, 837–844. [Google Scholar]
- Suzuki, T.; Hashimoto, S.; Toyoda, N.; Nagai, S.; Yamazaki, N.; Dong, H.Y.; Sakai, J.; Yamashita, T.; Nukiwa, T.; Matsushima, K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood 2000, 96, 2584–2591. [Google Scholar]
- Benz, K.; Breit, S.; Lukoschek, M.; Mau, H.; Richter, W. Molecular analysis of expansion, differentiation, and growth factor treatment of human chondrocytes identifies differentiation markers and growth-related genes. Biochem. Biophys. Res. Commun. 2002, 293, 284–292. [Google Scholar] [CrossRef]
- Stokes, D.G.; Liu, G.; Coimbra, I.B.; Piera-Velazquez, S.; Crowl, R.M.; Jimenez, S.A. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002, 46, 404–419. [Google Scholar] [CrossRef]
- Imabayashi, H.; Mori, T.; Gojo, S.; Kioyono, T.; Sugiyama, T.; Irie, R.; Isoga, T.; Hata, J.; Toyama, Y.; Umezawa, A. Redifferentiation of dedifferentiated chondrocytes and chondrogenesis of human bone marrow stromal cells via chondrosphere formation with expression profiling by large-scale cDNA analysis. Exp. Cell Res. 2003, 288, 35–50. [Google Scholar] [CrossRef]
- Junker, N.; Johansen, J.S.; Kristjansen, P.E.G.; Price, P.A. Monoclonal antibody therapy against YKL-40 delays growth in glioblastoma xenografts. In American Association for Cancer Research Annual Meeting: Proceedings 2007, Los Angeles, CA, USA, 14–18 April 2007. Abstract 4099.
- Pelloski, C.E.; Lin, E.; Zhang, L.; Yung, W.K.A.; Colman, H.; Liu, J.-L.; Woo, S.Y.; Heimberger, A.B.; Suki, D.; Prados, M.; Chang, S.; Barker, F.G., III; Fuller, G.N.; Aldape, K.D. Prognostic associations of activated mitogen-activated protein kinase and akt pathways in glioblastoma. Clin. Cancer Res. 2006, 12, 3935–3941. [Google Scholar] [CrossRef]
- Lee, C.G.; Haartl, D.; Lee, G.R.; Koller, B.; Matsuura, H.; Da Silva, C.A.; Sohn, M.H.; Cohn, L.; Homer, R.J.; Kozhich, A.A.; Humbles, A.; Keaney, J.; Coyle, A.; Chupp, G.; Reed, J.; Flavell, R.A.; Elias, J.A. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-12-induced tissue responses and apoposis. J. Exp. Med. 2009, 206, 1149–1166. [Google Scholar] [CrossRef]
- Shao, R.; Hamel, K.; Petersen, L.; Cao, Q.J.; Arenas, R.B.; Bigelow, C.; Bentley, B.; Yan, W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009, 28, 4456–4468. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumor vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef]
- Mizoguchi, E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006, 130, 398–411. [Google Scholar] [CrossRef]
- Mizoguchi, E.; Mizoguchi, A. Is the sugar always sweet in intestinal inflammation? Immunol. Res. 2007, 37, 47–60. [Google Scholar] [CrossRef]
- Kawada, M.; Chen, C.C.; Arihiro, A.; Nagatani, K.; Watanabe, T.; Mizoguchi, E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab. Invest. 2008, 88, 883–895. [Google Scholar] [CrossRef]
- Verheiden, G.F.M.; Rijnders, A.W.M.; Bos, E.; Coenen de-Roo, C.J.J.; van Staveren, C.J.; Miltenburg, A.M.M.; Meijerink, J.H.; Elewaut, D.; Keyser, F.; Veys, E.; Boots, A.M.H. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997, 40, 1115–1125. [Google Scholar] [CrossRef]
- Cope, A.P.; Patel, S.D.; Hall, F.; Congia, M.; Hubers, H.A.J.M.; Verheijden, G.F.; Boots, A.M.H.; Menon, R.; Trucco, M.; Rijnders, A.V.M.; Sønderstrup, G. T cell responses to a human cartilage autoantigen in the context of rheumatoid arthritis-associated and nonassociated HLA-DR4 alleles. Arthritis Rheum. 1999, 42, 1497–1507. [Google Scholar] [CrossRef]
- Joosten, L.A.B.; Coenen de-Roo, C.J.J.; Helsen, M.M.A.; Lubberts, E.; Boots, A.M.H.; van der Berg, W.B.; Miltenburg, A.M.M. Induction of tolerance with intranasal administration of human cartilage gp-39 in DBA/1 mice. Arthritis Rheum. 2000, 43, 645–655. [Google Scholar] [CrossRef]
- Vos, K.; Miltenburg, A.M.M.; van Meijgaarden, K.E.; van den Heuvel, M.; Elferink, D.G.; van Galen, P.J.M.; van Hogezand, R.A.; van Vilet-Daskalopoulou, E.; Ottenhoff, T.H.M.; Breedveld, F.C.; Boots, A.M.H.; de Vries, R.R.P. Cellular immune response to human cartilage glycoprotein-39 (HC gp-39)-derived peptides in rheumatoid arthritis and other inflammatory conditions. Rheumatology 2000, 39, 1326–1331. [Google Scholar] [CrossRef]
- Patil, N.S.; Hall, F.C.; Drover, S.; Spurrell, D.R.; Bos, E.; Cope, A.P.; Sonderstrup, G.; Mellins, E.D. Autoantigenic HCgp39 epitopes are presented by the HLA-DM-dependent presentation pathway in human B cells. J. Immunol. 2001, 166, 33–41. [Google Scholar]
- Kavanaugh, A.; Genovese, M.; Baughman, J.; Kivitz, A.; Bulpitt, K.; Olsen, N.; Weisman, M.; Matteson, E.; Furst, D.; van Vollenhoven, R.; Anderson, J.; Cohen, S.; Wei, N.; Meijerink, J.; Jacobs, C.; Mocci, S. Allele and antigen-specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase 1 trial. J. Rheumatol. 2003, 30, 449–454. [Google Scholar]
- Steenbakkers, P.G.A.; Baeten, D.; Rovers, E.; Veys, E.M.; Rijnders, A.W.M.; Meijerink, J.; Keyser, F.D.; Boots, A.M.H. Localization of MHC Class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J. Immunol. 2003, 170, 5719–5727. [Google Scholar]
- Dickey, B.F. Editorial. Exoskeletons and exhalation. N. Engl. J. Med. 2007, 357, 2082–2084. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Balkwill, F.; Coussens, L.M. An inflammatory link. Nature 2004, 431, 405–406. [Google Scholar] [CrossRef]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Hendrix, M.J.; Coussens, L.; Padarathsingh, M. Proteases, extracellular matrix, and cancer. Am. J. Pathol. 2004, 164, 1131–1139. [Google Scholar] [CrossRef]
- Moss, S.F.; Blaser, M.J. Mechanisms of disease: inflammation and the origins of cancer. Review. Nature Clin. Pract. 2005, 2, 90–97. [Google Scholar] [CrossRef]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Tlsty, T.D.; Coussens, L.M. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 119–150. [Google Scholar] [CrossRef]
- Lin, W.W.; Karin, M. A cytokine–mediated link between immunity, inflammation, and cancer. J. Clin. Invest. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- Gregoire, M.; Lieubeau, B. The role of fibroblasts in tumor behavior. Cancer Met. Rev. 1995, 14, 339–350. [Google Scholar] [CrossRef]
- Bissel, M.J.; Radisky, D. Putting tumors in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Kenny, P.A.; Bissell, M.J. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int. J. Cancer 2003, 107, 688–695. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Bigg, H.F.; Wait, R.; Rowan, A.D.; Cawston, T.E. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen fibril formation. J. Biol. Chem. 2006, 281, 21082–21095. [Google Scholar] [CrossRef]
- Jacques, C.; Recklies, A.D.; Levy, A.; Berenbaum, F. HC-gp39 contributes to chondrocyte differentiation by inducing SOX9 and type II collagen expressions. Osteoarthritis Cartilage. 2007, 15, 138–146. [Google Scholar] [CrossRef]
- Meyer, M.F.; Kreil, G. Cells expressing the DG42 gene from early Xenopus embryos synthesize hyaluronan. Proc. Natl. Acad. Sci. USA 1996, 93, 4543–4547. [Google Scholar] [CrossRef]
- Semino, C.E.; Specht, C.A.; Raimondi, A.; Robbins, P.W. Homologs of the Xenopus developmental gene DG42 are present in zebrafish and mouse and are involved in the synthesis of Nod-like chitin oligosaccharides during early embryogenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 4548–4553. [Google Scholar] [CrossRef]
- Varki, A. Does DG42 synthesize hyaluronan or chitin? A controversy about oligosaccharides in vertebrate development. Proc. Natl. Acad. Sci. USA 1996, 93, 4523–4525. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Fjeldstad, K.; Kolset, S.O. Decreasing the metastatic potential in cancers – targeting the heparan sulfate proteoglycans. Curr. Drug Targets 2005, 6, 665–682. [Google Scholar] [CrossRef]
- Iwata, T.; Kuwajima, M.; Sukeno, A.; Ishimaru, N.; Hayashi, Y.; Wabitsch, M.; Mizusawa, N.; Itakura, M.; Yoshimoto, K. YKL-40 secreted from adipose tissue inhibits degradation of type I collagen. Biochem. Biophys. Res. Commun. 2009, 388, 511–516. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; MacDonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef]
- Hayes, D.F.; Bast, R.C.; Desch, C.E.; Fritsche, H.; Kemeny, N.E.; Jessup, J.M.; Locker, G.Y.; Macdonald, J.S.; Mennel, R.G.; Norton, L.; Ravdin, P.; Taube, S.; Winn, R.J. Tumor Marker Utility Grading System: a framework to evaluate clinical utility of tumor markers. J. Natl. Cancer Inst. 1996, 88, 1456–1466. [Google Scholar] [CrossRef]
- Hayes, D.F. Determination of clinical utility of tumor markers: a tumor marker utility grading system. Recent Results Cancer Res. 1998, 152, 71–85. [Google Scholar] [CrossRef]
- Werner, M.; Fraser, C.; Silverberg, M. Clinical utility and validation of emerging biochemical markers for mammary adenocarcinoma. Clin. Chem. 1993, 39, 2386–2396. [Google Scholar]
- Morrow, D.A.; de Lemos, J.A. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007, 115, 949–952. [Google Scholar] [CrossRef]
- Freeman, W.M.; Bixler, G.V.; Brucklacher, R.M.; Lin, C.M.; Patel, K.M.; Vanguilder, H.D.; Lanoue, K.F.; Kimball, S.R.; Barber, A.J.; Antonetti, D.A.; Gardner, T.W.; Bronson, S.K. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics J. 2009. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schultz, N.A.; Johansen, J.S. YKL-40—A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients? Cancers 2010, 2, 1453-1491. https://doi.org/10.3390/cancers2031453
Schultz NA, Johansen JS. YKL-40—A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients? Cancers. 2010; 2(3):1453-1491. https://doi.org/10.3390/cancers2031453
Chicago/Turabian StyleSchultz, Nicolai A., and Julia S. Johansen. 2010. "YKL-40—A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients?" Cancers 2, no. 3: 1453-1491. https://doi.org/10.3390/cancers2031453
APA StyleSchultz, N. A., & Johansen, J. S. (2010). YKL-40—A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients? Cancers, 2(3), 1453-1491. https://doi.org/10.3390/cancers2031453