Multimodal Hazard Rate for Relapse in Breast Cancer: Quality of Data and Calibration of Computer Simulation
Abstract
:1. Introductory Comments on Quality of Breast Cancer Databases
2. Results and Discussion
What Other Databases Show a Multimodal Relapse Pattern?




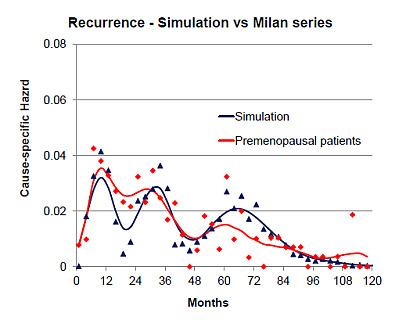
3. Can the Simulation Be Adjusted to Match Specific Clinical Data?


4. Discussion—Analysis and Synthesis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Retsky, M.; Demicheli, R.; Hrushesky, W.; Baum, M.; Gukas, I. Surgery triggers outgrowth of latent distant disease in breast cancer: An inconvenient truth? Cancers 2010, 2, 305–337. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, D.; Haybrittle, J.L. The curability of breast cancer. Lancet 1975, 2, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; Valagussa, P.; Moliterni, A.; Zambetti, M.; Brambilla, C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. N. Engl. J. Med. 1995, 332, 901–906. [Google Scholar] [CrossRef] [PubMed]
- McGuire, W.L.; Clark, G.M.; Fisher, E.R.; Henderson, I.C. Predicting recurrence and survival in breast cancer. A panel discussion. Breast Cancer Res. Treat. 1987, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Borg, A.; Tandon, A.K.; Sigurdsson, H.; Clark, G.M.; Fernö, M.; Fuqua, S.A.; Killander, D.; McGuire, W.L. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990, 50, 4332–4337. [Google Scholar] [PubMed]
- Demicheli, R.; Retsky, M.W.; Swartzendruber, D.E.; Bonadonna, G. Proposal for a new model of breast cancer metastatic development. Ann. Oncol. 1997, 8, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Retsky, M.W.; Demicheli, R.; Swartzendruber, D.E.; Bame, P.D.; Wardwell, R.H.; Bonadonna, G.; Speer, J.F.; Valagussa, P. Computer simulation of a breast cancer metastasis model. Breast Cancer Res. Treat. 1997, 45, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Vandenhende, J.; Berliere, M.; Machiels, J.P.; Nussbaum, B.; Legrand, C.; de Kock, M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010, 110, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- El Saghir, N.S.; Elhajj, I.I.; Geara, F.B.; Hourani, M.H. Trauma-associated growth of suspected dormant micrometastasis. BMC Cancer 2005, 5, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, F.S.; Rous, P. On the cause of the localization of secondary tumors at points of injury. J. Exp. Med. 1914, 20, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.D.; Rice, P.L.; Redente, E.F.; Kauvar, E.F.; Lemond, L.; Aly, T.; Wanebo, K.; Chan, E.D. Wound healing after trauma may predispose to lung cancer metastasis: Review of potential mechanisms. Am. J. Respir. Cell Mol. Biol. 2011, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.; Murphy, P.; Skipper, D. Preferential growth of blood-borne cancer cells at sites of trauma—A growth promoting role of macrophages. Adv. Exp. Med. Biol. 1988, 233, 245–251. [Google Scholar] [PubMed]
- Retsky, M. Comment: What would explain the sudden growth of lung cancer after minor trauma reported by El Saghir et al.? Available online: http://www.biomedcentral.com/1471-2407/5/94/comments/ (accessed on 18 November 2014).
- Retsky, M.W.; Demicheli, R.; Hrushesky, W.J.M.; Forget, P.; de Kock, M.; Gukas, I.; Rogers, R.A.; Baum, M.; Pachmann, K.; Vaidya, J.S. Promising development from translational or perhaps anti-translational research in breast cancer. Clin. Transl. Med. 2012, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Retsky, M.W.; Demicheli, R.; Hrushesky, W.J.M.; Forget, P.; DeKock, M.; Gukas, I.; Rogers, R.A.; Baum, M.; Sukhatme, V.; Vaidya, J.S. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: New findings and a review. Curr. Med. Chem. 2013, 20, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Kelsey, C.R. Recurrence dynamics for non-small-cell lung cancer: Effect of surgery on the development of metastases. J. Thorac. Oncol. 2012, 7, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Fornili, M.; Biganzoli, E. Bimodal mortality dynamics for uveal melanoma: A cue for metastasis development traits? BMC Cancer. 2014, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Machiels, J.P.; Coulie, P.G.; Berliere, M.; Poncelet, A.J.; Tombal, B.; Stainier, A.; Legrand, C.; Canon, J.L.; Kremer, Y.; et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann. Surg. Oncol. 2013, 20, S650–S660. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Bentin, C.; Machiels, J.P.; Berliere, M.; Coulie, P.G.; de Kock, M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br. J. Anaesth. 2014. [Google Scholar] [CrossRef]
- Bugada, D.; Allegri, M.; Lavandʼhomme, P.; de Kock, M.; Fanelli, G. Inflammation-based scores: A new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Baum, M. Does surgery disseminate or accelerate cancer? Lancet 1996, 347, 260. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Biganzoli, E.; Bonoldi, E.; Morabito, A.; Fanelli, M.; Boracchi, P. Angiogenesis sustains tumor dormancy in patients with breast cancer treated with adjuvant chemotherapy. Breast Cancer Res. Treat. 2001, 65, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, I.; Tsimelzon, A.; Weiss, H.; Clark, G.M.; Hilsenbeck, S.G. Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res. Treat. 2005, 89, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jerez, J.M.; Franci, L.; Alba, E.; Llombart-Cussiac, A.; Lluch, A.; Ribelles, N.; Munárriz, B.; Martín, M. Improvements of breast cancer prediction in high risk intervals using artificial neural networks. Breast Cancer Res. Treat. 2005, 94, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Karrison, T.G.; Ferguson, D.J.; Meier, P. Dormancy of mammary carcinoma after mastectomy. J. Natl. Cancer Inst. 1999, 91, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fortin, A.; Larochelle, M.; Laverdiere, J.; Lavertu, S.; Tremblay, D. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J. Clin. Oncol. 1999, 17, 101–109. [Google Scholar] [PubMed]
- Yakovlev, A.J.; Tsodikov, A.D.; Boucher, K.; Kerber, R. The shape of the hazard function in breast carcinoma. Cancer 1999, 85, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Gatta, G.; Micheli, A.; Verdecchia, A.; Capocaccia, R.; Crosignani, P.; Berrino, F. Survival and age at diagnosis of breast cancer in a population based cancer registry. Eur. J. Cancer 1991, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Stearns, A.T.; Hole, D.; George, W.D.; Kingsmore, D.B. Comparison of breast cancer mortality rates with those of ovarian and colorectal carcinoma. Br. J. Surg. 2007, 94, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tan, S.B.; Machin, D.; Wong, N.S. Confirmation of double-peaked time distribution of mortality among Asian breast cancer patients in a population-based study. Breast Cancer Res. 2007, 9, R21. [Google Scholar] [CrossRef]
- Langerod, A.; Zhao, H.; Borgan, O.; Nesland, J.M.; Bukholm, I.R.; Ikdahl, T.; Kåresen, R.; Børresen-Dale, A.L.; Jeffrey, S.S. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res. 2007, 9, R30. [Google Scholar] [CrossRef] [PubMed]
- Ripley, R.M.; Harris, A.L.; Tarassenko, L. Non-linear survival analysis using neural networks. Stat. Med. 2004, 23, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Jatoi, I.; Devesa, S.S. Assessing the impact of screening mammography: Breast cancer incidence and mortality rates in Connecticut (1943–2002). Breast Cancer Res. Treat. 2006, 99, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Miceli, R.; Moliterni, A.; Zambetti, M.; Hrushesky, W.J.; Retsky, M.W.; Valagussa, P.; Bonadonna, G. Breast cancer recurrence dynamics following adjuvant CMF is consistent with tumour dormancy and mastectomy-driven acceleration of the metastatic process. Ann. Oncol. 2005, 16, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Bonadonna, G.; Hrushesky, W.J.M.; Retsky, M.W.; Valagussa, P. Menopausal status dependence of the early mortality reduction due to diagnosing smaller breast cancers (T1 versus T2-T3): Relevance to screening. J. Clin. Oncol. 2004, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Fisher, E.R.; Redmond, C.; Wickerham, D.L.; Wolmark, N.; Mamounas, E.P.; Deutsch, M.; Margolese, R. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet 1991, 338, 327–331. [Google Scholar] [CrossRef] [PubMed]
- De la Rochefordiere, A.; Asselain, B.; Campana, F.; Scholl, S.M.; Fenton, J.; Vilcoq, J.R.; Durand, J.C.; Pouillart, P.; Magdelenat, H.; Fourquet, A. Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993, 341, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Marubini, E.; Del Vecchio, M.; Manzari, A.; Andreola, S.; Greco, M.; Luini, A.; Merson, M.; Saccozzi, R.; Rilke, F.; et al. Local recurrences and distant metastases after conservative breast cancer treatments: Partly independent events. J. Natl. Cancer Inst. 1995, 87, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Saphner, T.; Tormey, D.C.; Gray, R. Annual hazard rates of recurrence for breast cancer after primary therapy. J. Clin. Oncol. 1996, 14, 2738–2746. [Google Scholar] [PubMed]
- Hilsenbeck, S.G.; Ravdin, P.M.; de Moor, C.A.; Chamness, G.C.; Osborne, C.K.; Clark, G.M. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res. Treat. 1998, 52, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Sass, R.; Fisher, B. Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer 1984, 53, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Bedwinek, J. Adjuvant irradiation for early breast cancer. An on-going controversy. Cancer 1984, 53, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Dukic, V.; Dignam, J. Bayesian hierarchical multiresolution hazard model for the study of timedependent failure patterns in early stage breast cancer. Bayesian Anal. 2007, 2, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Yanagita, Y.; Fujisawa, T.; Koida, T. Study of time-course changes in annual recurrence rates for breast cancer: Data analysis of 2,209 patients for 10 years post-surgery. Breast Cancer Res. Treat. 2007, 106, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Di, G.; Zhou, L.; Lu, J.; Liu, G.; Wu, J.; Shen, K.; Han, Q.; Shen, Z.; Shao, Z. Time-varying pattern of recurrence risk for Chinese breast cancer patients. Breast Cancer Res. Treat. 2009, 114, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ribelles, N.; Perez-Villa, L.; Jerez, J.M.; Pajares, B.; Vicioso, L.; Jimenez, B.; de Luque, V.; Franco, L.; Gallego, E.; Marquez, A.; et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 2013, 15, R98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, D.; Fenstermacher, D. A. Integrated analysis of independent gene expression microarray datasets improves the predictability of breast cancer outcome. BMC Genomics 2007, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Miceli, R.; Valagussa, P.; Bonadonna, G. Re: Dormancy of mammary carcinoma after mastectomy. J. Natl. Cancer Inst. 2000, 92, 347–348. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retsky, M.; Demicheli, R. Multimodal Hazard Rate for Relapse in Breast Cancer: Quality of Data and Calibration of Computer Simulation. Cancers 2014, 6, 2343-2355. https://doi.org/10.3390/cancers6042343
Retsky M, Demicheli R. Multimodal Hazard Rate for Relapse in Breast Cancer: Quality of Data and Calibration of Computer Simulation. Cancers. 2014; 6(4):2343-2355. https://doi.org/10.3390/cancers6042343
Chicago/Turabian StyleRetsky, Michael, and Romano Demicheli. 2014. "Multimodal Hazard Rate for Relapse in Breast Cancer: Quality of Data and Calibration of Computer Simulation" Cancers 6, no. 4: 2343-2355. https://doi.org/10.3390/cancers6042343
APA StyleRetsky, M., & Demicheli, R. (2014). Multimodal Hazard Rate for Relapse in Breast Cancer: Quality of Data and Calibration of Computer Simulation. Cancers, 6(4), 2343-2355. https://doi.org/10.3390/cancers6042343