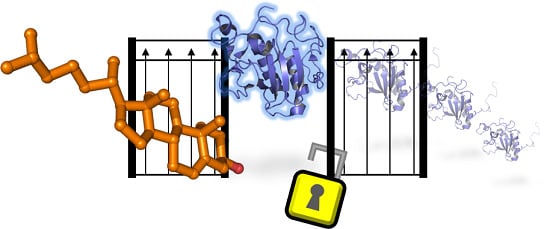
Hedgehog Cholesterolysis: Specialized Gatekeeper to Oncogenic Signaling
Abstract
:1. Introduction

2. Cholesterolysis—Liberating the Ligand


2.1. The HINT Domain from Drosophila Melanogaster Hh Protein

2.2. Biological Role of Hh Ligand Cholesteroylation

3. Targeting Cholesterolysis—A Ligand Deprivation Approach for Hh Ligand Driven Cancers
3.1. Target Attributes that Mitigate Risk
3.1.1. Decisive Role Played by Cholesterolysis in Hh Signaling
3.1.2. Upstream Target in the Pathway
3.1.3. A Transformation Unique to Hedgehog
3.2. Assays for Cholesterolysis Amenable to High Throughput Screening

3.3. Endogenous Regulators of Hh Cholesterolysis

4. Conclusions and Outlook
|
Acknowledgments
Conflicts of Interest
References
- Briscoe, J.; Therond, P.P. The mechanisms of hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.V.; Scott, M.P. Hedgehog and patched in neural development and disease. Neuron 1998, 21, 1243–1257. [Google Scholar] [CrossRef]
- Harmon, E.B.; Ko, A.H.; Kim, S.K. Hedgehog signaling in gastrointestinal development and disease. Curr. Mol. Med. 2002, 2, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hui, C.C. Hedgehog signaling in development and cancer. Dev. Cell 2008, 15, 801–812. [Google Scholar] [CrossRef] [PubMed]
- VanHook, A.M. Focus issue: Fine-tuning hedgehog signaling in development and disease. Sci. Signal. 2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Dakubo, G.; Howley, P.; Campsall, K.D.; Mazarolle, C.J.; Shiga, S.A.; Lewis, P.M.; McMahon, A.P.; Wallace, V.A. Development of normal retinal organization depends on sonic hedgehog signaling from ganglion cells. Nat. Neurosci. 2002, 5, 831–832. [Google Scholar] [PubMed]
- Parkin, C.A.; Ingham, P.W. The adventures of sonic hedgehog in development and repair. I. Hedgehog signaling in gastrointestinal development and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G363–G367. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhou, Y.; Beachy, P.A.; Moses, K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing drosophila eye. Cell 1993, 75, 927–938. [Google Scholar] [PubMed]
- Roessler, E.; Belloni, E.; Gaudenz, K.; Vargas, F.; Scherer, S.W.; Tsui, L.C.; Muenke, M. Mutations in the C-terminal domain of sonic hedgehog cause holoprosencephaly. Hum. Mol. Genet. 1997, 6, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Belloni, E.; Gaudenz, K.; Jay, P.; Berta, P.; Scherer, S.W.; Tsui, L.C.; Muenke, M. Mutations in the human sonic hedgehog gene cause holoprosencephaly. Nat. Genet. 1996, 14, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.; Hui, C.C. Hedgehog signaling and congenital malformations. Clin. Genet. 2005, 67, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, A.; Isebaert, S.; Haustermans, K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int. J. Mol. Sci. 2013, 14, 13979–14007. [Google Scholar] [CrossRef] [PubMed]
- Sims-Mourtada, J.; Yang, D.; Tworowska, I.; Larson, R.; Smith, D.; Tsao, N.; Opdenaker, L.; Mourtada, F.; Woodward, W. Detection of canonical hedgehog signaling in breast cancer by 131-iodine-labeled derivatives of the sonic hedgehog protein. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.L.; Sanchez-Mejias, A.; Wang, Z.; Flaveny, C.; Long, J.; Singh, S.; Rodriguez-Blanco, J.; Tokhunts, R.; Giambelli, C.; Briegel, K.J.; et al. Hedgehog signaling regulates bladder cancer growth and tumorigenicity. Cancer Res. 2012, 72, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Feuerstein, M.A.; Levina, E.; Baghel, P.S.; Carkner, R.D.; Tanner, M.J.; Shtutman, M.; Vacherot, F.; Terry, S.; de la Taille, A.; et al. Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol. Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Zunich, S.M.; Douglas, T.; Valdovinos, M.; Chang, T.; Bushman, W.; Walterhouse, D.; Iannaccone, P.; Lamm, M.L. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol. Cancer 2009. [Google Scholar] [CrossRef] [PubMed]
- Scales, S.J.; de Sauvage, F.J. Mechanisms of hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 2009, 30, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Di Magliano, M.P.; Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 2003, 3, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, J.W.; de Sauvage, F.J. Paracrine hedgehog signaling in cancer. Cancer Res. 2009, 69, 6007–6010. [Google Scholar] [CrossRef] [PubMed]
- Low, J.A.; de Sauvage, F.J. Clinical experience with hedgehog pathway inhibitors. J. Clin. Oncol. 2010, 28, 5321–5326. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L.; de Sauvage, F.J. Targeting the hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006, 5, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Guha, M. Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat. Rev. Drug Discov. 2012, 11, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Low, J.A.; Marsters, J.C., Jr.; Robarge, K.; Rubin, L.L.; de Sauvage, F.J.; Sutherlin, D.P.; Wong, H.; Yauch, R.L. Discovery and preclinical development of vismodegib. Expert Opin. Drug Discov. 2014, 9, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Taipale, J.; Cooper, M.K.; Beachy, P.A. Inhibition of hedgehog signaling by direct binding of cyclopamine to smoothened. Genes Dev. 2002, 16, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Gaffield, W.; Kapur, R.P.; Roelink, H. The teratogenic veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 1998, 125, 3553–3562. [Google Scholar] [PubMed]
- Gould, A.; Missailidis, S. Targeting the hedgehog pathway: The development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev. Med. Chem. 2011, 11, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Heretsch, P.; Tzagkaroulaki, L.; Giannis, A. Cyclopamine and hedgehog signaling: Chemistry, biology, medical perspectives. Angew. Chem. Int. Ed. Engl. 2010, 49, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Struhl, G. Dual roles for patched in sequestering and transducing hedgehog. Cell 1996, 87, 553–563. [Google Scholar] [CrossRef]
- Hahn, H.; Christiansen, J.; Wicking, C.; Zaphiropoulos, P.G.; Chidambaram, A.; Gerrard, B.; Vorechovsky, I.; Bale, A.E.; Toftgard, R.; Dean, M.; et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J. Biol. Chem. 1996, 271, 12125–12128. [Google Scholar] [CrossRef] [PubMed]
- Marigo, V.; Davey, R.A.; Zuo, Y.; Cunningham, J.M.; Tabin, C.J. Biochemical evidence that patched is the hedgehog receptor. Nature 1996, 384, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Struhl, G. In vivo evidence that patched and smoothened constitute distinct binding and transducing components of a hedgehog receptor complex. Development 1998, 125, 4943–4948. [Google Scholar] [PubMed]
- Zhu, H.; Carpenter, R.L.; Han, W.; Lo, H.W. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett. 2014, 343, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; Ruiz i Altaba, A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lauth, M.; Bergstrom, A.; Shimokawa, T.; Toftgard, R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA 2007, 104, 8455–8460. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.J.; Gardner, D.; Beachy, P.A. Arsenic antagonizes the hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl. Acad. Sci. USA 2010, 107, 13432–13437. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.L.; Li, H.; Kozul, C.D.; Black, K.E.; Singh, S.; Gosse, J.A.; DiRenzo, J.; Martin, K.A.; Wang, B.; Hamilton, J.W.; et al. Activation of hedgehog signaling by the environmental toxicant arsenic may contribute to the etiology of arsenic-induced tumors. Cancer Res. 2010, 70, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol modification of hedgehog signaling proteins in animal development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ekker, S.C.; von Kessler, D.P.; Porter, J.A.; Sun, B.I.; Beachy, P.A. Autoproteolysis in hedgehog protein biogenesis. Science 1994, 266, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Bumcrot, D.A.; Takada, R.; McMahon, A.P. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol. Cell Biol. 1995, 15, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.K. Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc. Natl. Acad. Sci. USA 2000, 97, 7307–7312. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tukachinsky, H.; Huang, C.H.; Jao, C.; Chu, Y.R.; Tang, H.Y.; Mueller, B.; Schulman, S.; Rapoport, T.A.; Salic, A. Processing and turnover of the hedgehog protein in the endoplasmic reticulum. J. Cell Biol. 2011, 192, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Pepinsky, R.B.; Zeng, C.; Wen, D.; Rayhorn, P.; Baker, D.P.; Williams, K.P.; Bixler, S.A.; Ambrose, C.M.; Garber, E.A.; Miatkowski, K.; et al. Identification of a palmitic acid-modified form of human sonic Hedgehog. J. Biol. Chem. 1998, 273, 14037–14045. [Google Scholar] [CrossRef] [PubMed]
- Buglino, J.A.; Resh, M.D. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 2008, 283, 22076–22088. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, Z.; Mann, R.K.; Nellen, D.; von Kessler, D.P.; Bellotto, M.; Beachy, P.A.; Basler, K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 2001, 293, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Buglino, J.A.; Resh, M.D. Palmitoylation of hedgehog proteins. Vitam. Horm. 2012, 88, 229–252. [Google Scholar] [PubMed]
- Perler, F.B. Protein splicing of inteins and hedgehog autoproteolysis: Structure, function, and evolution. Cell 1998, 92, 1–4. [Google Scholar] [CrossRef]
- Paulus, H. Protein splicing and related forms of protein autoprocessing. Annu. Rev. Biochem. 2000, 69, 447–496. [Google Scholar] [CrossRef] [PubMed]
- Tukachinsky, H.; Kuzmickas, R.P.; Jao, C.Y.; Liu, J.; Salic, A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Von Kessler, D.P.; Ekker, S.C.; Young, K.E.; Lee, J.J.; Moses, K.; Beachy, P.A. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 1995, 374, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.K.; Beachy, P.A. Cholesterol modification of proteins. Biochim. Biophys. Acta 2000, 1529, 188–202. [Google Scholar] [CrossRef]
- Ciepla, P.; Konitsiotis, A.D.; Serwa, R.A.; Masumoto, N.; Leong, W.P.; Dallman, M.J.; Magee, A.I.; Tate, E.W. New chemical probes targeting cholesterylation of sonic hedgehog in human cells and zebrafish. Chem. Sci. 2014, 5, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.M.; Porter, J.A.; Young, K.E.; Koonin, E.V.; Beachy, P.A.; Leahy, D.J. Crystal structure of a hedgehog autoprocessing domain: Homology between hedgehog and self-splicing proteins. Cell 1997, 91, 85–97. [Google Scholar] [CrossRef]
- Vincent, S.; Thomas, A.; Brasher, B.; Benson, J.D. Targeting of proteins to membranes through hedgehog auto-processing. Nat. Biotechnol. 2003, 21, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Ekker, S.C.; Park, W.J.; von Kessler, D.P.; Young, K.E.; Chen, C.H.; Ma, Y.; Woods, A.S.; Cotter, R.J.; Koonin, E.V.; et al. Hedgehog patterning activity: Role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996, 86, 21–34. [Google Scholar] [CrossRef]
- Lewis, C.A., Jr.; Wolfenden, R. Amide bonds to the nitrogen atoms of cysteine and serine as "weak points" in the backbones of proteins. Biochemistry 2011, 50, 7259–7264. [Google Scholar] [CrossRef] [PubMed]
- Johansson, D.G.; Wallin, G.; Sandberg, A.; Macao, B.; Aqvist, J.; Hard, T. Protein autoproteolysis: Conformational strain linked to the rate of peptide cleavage by the pH dependence of the N –> O acyl shift reaction. J. Am. Chem. Soc. 2009, 131, 9475–9477. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, A.; Shekhtman, A.; Cowburn, D.; Muir, T.W. Semisynthesis of a segmental isotopically labeled protein splicing precursor: NMR evidence for an unusual peptide bond at the N-extein-intein junction. Proc. Natl. Acad. Sci. USA 2004, 101, 6397–6402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.P.; Topilina, N.I.; Stanger, M.J.; Van Roey, P.; Belfort, M. Structure of catalytically competent intein caught in a redox trap with functional and evolutionary implications. Nat. Struct. Mol. Biol. 2011, 18, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Perler, F.B.; Xu, M.-Q.; Paulus, H. Protein splicing and autoproteolysis mechanisms. Curr. Opin. Chem. Biol. 1997, 1, 292–299. [Google Scholar] [CrossRef]
- Dearden, A.K.; Callahan, B.; Roey, P.V.; Li, Z.; Kumar, U.; Belfort, M.; Nayak, S.K. A conserved threonine spring-loads precursor for intein splicing. Protein Sci. 2013, 22, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, J.; Albracht, C.D.; Hsu, A.; Chen, W.; Marieni, M.D.; Colelli, K.M.; Williams, J.E.; Reitter, J.N.; Mills, K.V.; et al. Structural and mutational studies of a hyperthermophilic intein from DNA polymerase ii of pyrococcus abyssi. J. Biol. Chem. 2011, 286, 38638–38648. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, Y.; Ban, D.; Lopez, M.M.; Belfort, M.; Wang, C. Backbone dynamics and global effects of an activating mutation in minimized Mtu RecA inteins. J. Mol. Biol. 2010, 400, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Binschik, J.; Mootz, H.D. Chemical bypass of intein-catalyzed N-S acyl shift in protein splicing. Angew. Chem. Int. Ed. Engl. 2013, 52, 4260–4264. [Google Scholar] [CrossRef] [PubMed]
- Van Roey, P.; Pereira, B.; Li, Z.; Hiraga, K.; Belfort, M.; Derbyshire, V. Crystallographic and mutational studies of mycobacterium tuberculosis recA mini-inteins suggest a pivotal role for a highly conserved aspartate residue. J. Mol. Biol. 2007, 367, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, M.C.; Xega, K.; Li, L.; Xie, J.; Wang, C.; Linhardt, R.J.; Reitter, J.N.; Mills, K.V. Internal disulfide bond acts as a switch for intein activity. Biochemistry 2013, 52, 5920–5927. [Google Scholar] [CrossRef] [PubMed]
- Topilina, N.I.; Green, C.M.; Jayachandran, P.; Kelley, D.S.; Stanger, M.J.; Piazza, C.L.; Nayak, S.; Belfort, M. SufB intein of Mycobacterium tuberculosis as a sensor for oxidative and nitrosative stresses. Proc. Natl. Acad. Sci. USA 2015, 112, 10348–10353. [Google Scholar] [CrossRef] [PubMed]
- Riobo, N.A. Cholesterol and its derivatives in sonic hedgehog signaling and cancer. Curr. Opin. Pharmacol. 2012, 12, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; McMahon, A.P. Cholesterol modification of hedgehog family proteins. J. Clin. Investig. 2002, 110, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A. Hedgehog morphogen: From secretion to reception. Trends Cell Biol. 2011, 21, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A.; Ruel, L.; Staccini-Lavenant, L.; Therond, P.P. Cholesterol modification is necessary for controlled planar long-range activity of hedgehog in Drosophila epithelia. Development 2006, 133, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, I.; Chiang, C. A conserved mechanism of hedgehog gradient formation by lipid modifications. Trends Cell Biol. 2007, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S. Multiple roles for lipids in the hedgehog signalling pathway. Nat. Rev. Mol. Cell Biol. 2008, 9, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.K.; Valadez, J.G.; Bowman, A.B.; Cooper, M.K. Lipid modifications of sonic hedgehog ligand dictate cellular reception and signal response. PLoS ONE 2011, 6, e21353. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Wolf, A.; Wagner, M.; Kuhlmann, J.; Waldmann, H. The cholesterol membrane anchor of the hedgehog protein confers stable membrane association to lipid-modified proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 8531–8536. [Google Scholar] [CrossRef] [PubMed]
- Teruya, K.; Nishizawa, K.; Doh-ura, K. Semisynthesis of a protein with cholesterol at the C-terminal, targeted to the cell membrane of live cells. Protein J. 2010, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ingallinella, P.; Bianchi, E.; Ladwa, N.A.; Wang, Y.J.; Hrin, R.; Veneziano, M.; Bonelli, F.; Ketas, T.J.; Moore, J.P.; Miller, M.D.; et al. Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc. Natl. Acad. Sci. USA 2009, 106, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Schneider, A.; Schlechtingen, G.; Weidlich, S.; Ries, J.; Braxmeier, T.; Schwille, P.; Schulz, J.B.; Schroeder, C.; Simons, M.; et al. Efficient inhibition of the Alzheimer's disease beta-secretase by membrane targeting. Science 2008, 320, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Antos, J.M.; Miller, G.M.; Grotenbreg, G.M.; Ploegh, H.L. Lipid modification of proteins through sortase-catalyzed transpeptidation. J. Am. Chem. Soc. 2008, 130, 16338–16343. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.P.; Taylor, F.R.; Pepinsky, R.B. Purifying the hedgehog protein and its variants. Methods Mol. Biol. 2007, 397, 1–22. [Google Scholar] [PubMed]
- Taylor, F.R.; Wen, D.; Garber, E.A.; Carmillo, A.N.; Baker, D.P.; Arduini, R.M.; Williams, K.P.; Weinreb, P.H.; Rayhorn, P.; Hronowski, X.; et al. Enhanced potency of human sonic hedgehog by hydrophobic modification. Biochemistry 2001, 40, 4359–4371. [Google Scholar] [CrossRef] [PubMed]
- Creanga, A.; Glenn, T.D.; Mann, R.K.; Saunders, A.M.; Talbot, W.S.; Beachy, P.A. Scube/you activity mediates release of dually lipid-modified hedgehog signal in soluble form. Genes Dev. 2012, 26, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Diehl, A.M.; Li, Y.X. Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Lab. Investig. 2009, 89, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Panakova, D.; Sprong, H.; Marois, E.; Thiele, C.; Eaton, S. Lipoprotein particles are required for hedgehog and wingless signalling. Nature 2005, 435, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Goetz, J.A.; Suber, L.M.; Scott, W.J., Jr.; Schreiner, C.M.; Robbins, D.J. A freely diffusible form of sonic hedgehog mediates long-range signalling. Nature 2001, 411, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Singh, S.; Schilling, N.S.; Robbins, D.J. The adventures of sonic hedgehog in development and repair. III. Hedgehog processing and biological activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G844–G849. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Goswami, D.; Manonmani, A.; Sharma, P.; Ranganath, H.A.; VijayRaghavan, K.; Shashidhara, L.S.; Sowdhamini, R.; Mayor, S. Nanoscale organization of hedgehog is essential for long-range signaling. Cell 2008, 133, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; White, B.; Tyurina, O.V.; Guner, B.; Larson, T.; Lee, H.Y.; Karlstrom, R.O.; Kohtz, J.D. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development 2004, 131, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; Swierczynska, M.M.; Kumari, V.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Eaton, S. Secretion and signaling activities of lipoprotein-associated hedgehog and non-sterol-modified hedgehog in flies and mammals. PLoS Biol. 2013, 11, e1001505. [Google Scholar] [CrossRef] [PubMed]
- Traiffort, E.; Dubourg, C.; Faure, H.; Rognan, D.; Odent, S.; Durou, M.R.; David, V.; Ruat, M. Functional characterization of sonic hedgehog mutations associated with holoprosencephaly. J. Biol. Chem. 2004, 279, 42889–42897. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.M.; Dunn, M.P.; McMahon, J.A.; Logan, M.; Martin, J.F.; St-Jacques, B.; McMahon, A.P. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 2001, 105, 599–612. [Google Scholar] [CrossRef]
- Yauch, R.L.; Gould, S.E.; Scales, S.J.; Tang, T.; Tian, H.; Ahn, C.P.; Marshall, D.; Fu, L.; Januario, T.; Kallop, D.; et al. A paracrine requirement for hedgehog signalling in cancer. Nature 2008, 455, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Mas, C.; Ruiz i Altaba, A. Interference with HH-GLI signaling inhibits prostate cancer. Trends Mol. Med. 2005, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Carkner, R.; Buttyan, R. The hedgehog/Gli signaling paradigm in prostate cancer. Expert Rev. Endocrinol. Metab 2011, 6, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.C.; Joyner, A.L. Hedgehog signaling in prostate epithelial-mesenchymal growth regulation. Dev. Biol. 2015, 400, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pepicelli, C.V.; Dibble, C.C.; Catbagan, W.; Zarycki, J.L.; Laciak, R.; Gipp, J.; Shaw, A.; Lamm, M.L.; Munoz, A.; et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 2004, 145, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Hernandez, A.M.; Stecca, B.; Kahler, A.J.; DeGueme, A.M.; Barrett, A.; Beyna, M.; Datta, M.W.; Datta, S.; Ruiz i Altaba, A. Inhibition of prostate cancer proliferation by interference with SONICHEDGEHOG-GLI1 signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 12561–12566. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, N.; Ghaffari, M.; Pandey, M.; Iu, I.; Fazli, L.; Kashiwagi, M.; Tojo, H.; Nakanishi, O.; Gleave, M.E.; Cox, M.E. Tak-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling. Int. J. Cancer 2013, 133, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.M.; Xu, D.; Cao, Y.; Kabraji, S.; Allen, D.; Kersemans, V.; Beech, J.; Smart, S.; Hamdy, F.; Ishkanian, A.; et al. Protease nexin 1 inhibits hedgehog signaling in prostate adenocarcinoma. J. Clin. Investig. 2012, 122, 4025–4036. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Gipp, J.; Bushman, W. The sonic hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene 2009, 28, 4480–4490. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, notch, and hedgehog pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Bjerregaard, R.; Bushman, W.; Beebe, D.J. Hedgehog signaling in myofibroblasts directly promotes prostate tumor cell growth. Integr. Biol. 2012, 4, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tang, T.; Eastham-Anderson, J.; Dunlap, D.; Alicke, B.; Nannini, M.; Gould, S.; Yauch, R.; Modrusan, Z.; DuPree, K.J.; et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc. Natl. Acad. Sci. USA 2011, 108, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Levina, E.; Chen, M.; Carkner, R.; Shtutman, M.; Buttyan, R. Paracrine hedgehog increases the steroidogenic potential of prostate stromal cells in a Gli-dependent manner. Prostate 2012, 72, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tanner, M.; Levine, A.C.; Levina, E.; Ohouo, P.; Buttyan, R. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell Cycle 2009, 8, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; LaPensee, C.R.; Krill, K.T.; Hammer, G.D. Hedgehog signaling and steroidogenesis. Annu. Rev. Physiol. 2015, 77, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Sudo, S.; Aad, P.Y.; Wang, L.S.; Chun, S.Y.; Ben-Shlomo, I.; Klein, C.; Hsueh, A.J. The hedgehog-patched signaling pathway and function in the mammalian ovary: A novel role for hedgehog proteins in stimulating proliferation and steroidogenesis of theca cells. Reproduction 2009, 138, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Theodoropoulou, M.; Stalla, J.; Tonn, J.C.; Losa, M.; Renner, U.; Stalla, G.K.; Paez-Pereda, M. Expression and function of Sonic Hedgehog pathway components in pituitary adenomas: Evidence for a direct role in hormone secretion and cell proliferation. J. Clin. Endocrinol. MeTable 2005, 90, 6687–6694. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Pan, Y.; Luo, H.; Xiong, W.; Zhu, H.; Ruan, H.; Wang, J.; Zou, C.; Tang, L.; Iguchi, T.; et al. Hedgehog signaling stimulates the conversion of cholesterol to steroids. Cell Signal 2015, 27, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O. Combination therapy: Abiraterone prolongs survival in metastatic prostate cancer. Nat. Rev. Clin. Oncol. 2011, 8, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Hampton, R.Y. Er-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 2002, 14, 476–482. [Google Scholar] [CrossRef]
- Tokhunts, R.; Singh, S.; Chu, T.; D'Angelo, G.; Baubet, V.; Goetz, J.A.; Huang, Z.; Yuan, Z.; Ascano, M.; Zavros, Y.; et al. The full-length unprocessed hedgehog protein is an active signaling molecule. J. Biol. Chem. 2010, 285, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, C.A.; Asp, E.; Emerson, C.P., Jr. A new role for hedgehogs in juxtacrine signaling. Mech. Dev. 2014, 131, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Maun, H.R.; Wen, X.; Lingel, A.; de Sauvage, F.J.; Lazarus, R.A.; Scales, S.J.; Hymowitz, S.G. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J. Biol. Chem. 2010, 285, 26570–26580. [Google Scholar] [CrossRef] [PubMed]
- O'Toole, S.A.; Machalek, D.A.; Shearer, R.F.; Millar, E.K.; Nair, R.; Schofield, P.; McLeod, D.; Cooper, C.L.; McNeil, C.M.; McFarland, A.; et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011, 71, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E.; Rios-Esteves, J.; Ouerfelli, O.; Glickman, J.F.; Resh, M.D. Inhibitors of hedgehog acyltransferase block sonic hedgehog signaling. Nat. Chem. Biol. 2013, 9, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E.; Matevossian, A.; Resh, M.D. Hedgehog acyltransferase as a target in pancreatic ductal adenocarcinoma. Oncogene 2015, 34, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Matevossian, A.; Resh, M.D. Hedgehog acyltransferase as a target in estrogen receptor positive, HER2 amplified, and tamoxifen resistant breast cancer cells. Mol. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chu, Y.R.; Ye, Y.; Chen, X. Role of herp and a herp-related protein in HRD1-dependent protein degradation at the endoplasmic reticulum. J. Biol. Chem. 2014, 289, 4444–4454. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsiao, H.T.; Chu, Y.R.; Ye, Y.; Chen, X. Derlin2 protein facilitates HRD1-mediated retro-translocation of sonic hedgehog at the endoplasmic reticulum. J. Biol. Chem. 2013, 288, 25330–25339. [Google Scholar] [CrossRef] [PubMed]
- Heal, W.P.; Jovanovic, B.; Bessin, S.; Wright, M.H.; Magee, A.I.; Tate, E.W. Bioorthogonal chemical tagging of protein cholesterylation in living cells. Chem. Commun. 2011, 47, 4081–4083. [Google Scholar] [CrossRef] [PubMed]
- Hulce, J.J.; Cognetta, A.B.; Niphakis, M.J.; Tully, S.E.; Cravatt, B.F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 2013, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gianoulis, T.A.; Yip, K.Y.; Gerstein, M.; Snyder, M. Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell 2010, 143, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Doles, J.; Cook, C.; Shi, X.; Valosky, J.; Lipinski, R.; Bushman, W. Functional compensation in hedgehog signaling during mouse prostate development. Dev. Biol. 2006, 295, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Q.; Paulus, H. A high-throughput, homogeneous, fluorescence polarization assay for inhibitors of hedgehog protein autoprocessing. J. Biomol. Screen. 2010, 15, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.S.; Ngoje, G.; Lageman, T.J.; Bordeau, B.M.; Belfort, M.; Callahan, B.P. Forster resonance energy transfer-based cholesterolysis assay identifies a novel hedgehog inhibitor. Anal. Biochem. 2015, 488, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Johannes, L.; Goud, B.; Antony, C.; Lingwood, C.A.; Daneman, R.; Grinstein, S. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl. Acad. Sci. USA 1998, 95, 2997–3002. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Eryilmaz, E.; Cowburn, D.; Muir, T.W. Extein residues play an intimate role in the rate-limiting step of protein trans-splicing. J. Am. Chem. Soc. 2013, 135, 5839–5847. [Google Scholar] [CrossRef] [PubMed]
- Amitai, G.; Callahan, B.P.; Stanger, M.J.; Belfort, G.; Belfort, M. Modulation of intein activity by its neighboring extein substrates. Proc. Natl. Acad. Sci. USA 2009, 106, 11005–11010. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Shemella, P.T.; Amitai, G.; Belfort, G.; Nayak, S.K.; Belfort, M. Spontaneous proton transfer to a conserved intein residue determines on-pathway protein splicing. J. Mol. Biol. 2011, 406, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.W.; Camarero, J.A. Intein applications: From protein purification and labeling to metabolic control methods. J. Biol. Chem. 2014, 289, 14512–14519. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.S.; Xie, X.J.; Laraway, B.; Ngoje, G.; Wang, C.; Callahan, B.P. Active site targeting of hedgehog precursor protein with phenylarsine oxide. Chembiochem 2015, 16, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Owen, T.; Xia, K.; Singh, A.V.; Tou, E.; Li, L.; Arduini, B.; Li, H.; Wan, L.Q.; Callahan, B.; et al. Zinc inhibits hedgehog autoprocessing: Linking zinc deficiency with hedgehog activation. J. Biol. Chem. 2015, 290, 11591–11600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.; Xi, Z.; Luo, Z.; Xu, X.; Wang, C.; Liu, Y. Metal ions binding to reca inteins from mycobacterium tuberculosis. Mol. Biosyst. 2009, 5, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ye, S.; Ferrandon, S.; Evans, T.C.; Xu, M.Q.; Rao, Z. Crystal structures of an intein from the split dnae gene of synechocystis sp. Pcc6803 reveal the catalytic model without the penultimate histidine and the mechanism of zinc ion inhibition of protein splicing. J. Mol. Biol. 2005, 353, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Poland, B.W.; Xu, M.Q.; Quiocho, F.A. Structural insights into the protein splicing mechanism of PI-Scei. J. Biol. Chem. 2000, 275, 16408–16413. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Masarik, M.; Adam, V.; Eckschlager, T.; Provaznik, I.; Kizek, R. Serum and tissue zinc in epithelial malignancies: A meta-analysis. PLoS ONE 2014, 9, e99790. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.U.; Kazi, T.G.; Afridi, H.I.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Syed, N. Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clin. Chim. Acta 2007, 379, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Karhadkar, S.S.; Bova, G.S.; Abdallah, N.; Dhara, S.; Gardner, D.; Maitra, A.; Isaacs, J.T.; Berman, D.M.; Beachy, P.A. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004, 431, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Sheng, T.; Li, C.; Zhang, X.; Chi, S.; He, N.; Chen, K.; McCormick, F.; Gatalica, Z.; Xie, J. Activation of the hedgehog pathway in advanced prostate cancer. Mol. Cancer 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, D.N.; Berman, D.M.; Baylin, S.B. Hedgehog signaling: Progenitor phenotype in small-cell lung cancer. Cell Cycle 2003, 2, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Govindan, R. Hedgehog signaling pathway and lung cancer. J. Thorac. Oncol. 2007, 2, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Horiuchi, A.; Kikuchi, N.; Osada, R.; Yoshida, J.; Shiozawa, T.; Konishi, I. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: It's inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007, 98, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Siu, M.K.; Au, C.W.; Wong, E.S.; Chan, H.Y.; Ip, P.P.; Ngan, H.Y.; Cheung, A.N. Aberrant activation of hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis 2009, 30, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yauch, R.L.; Dijkgraaf, G.J.; Alicke, B.; Januario, T.; Ahn, C.P.; Holcomb, T.; Pujara, K.; Stinson, J.; Callahan, C.A.; Tang, T.; et al. Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science 2009, 326, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Amakye, D.; Jagani, Z.; Dorsch, M. Unraveling the therapeutic potential of the hedgehog pathway in cancer. Nat. Med. 2013, 19, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callahan, B.P.; Wang, C. Hedgehog Cholesterolysis: Specialized Gatekeeper to Oncogenic Signaling. Cancers 2015, 7, 2037-2053. https://doi.org/10.3390/cancers7040875
Callahan BP, Wang C. Hedgehog Cholesterolysis: Specialized Gatekeeper to Oncogenic Signaling. Cancers. 2015; 7(4):2037-2053. https://doi.org/10.3390/cancers7040875
Chicago/Turabian StyleCallahan, Brian P., and Chunyu Wang. 2015. "Hedgehog Cholesterolysis: Specialized Gatekeeper to Oncogenic Signaling" Cancers 7, no. 4: 2037-2053. https://doi.org/10.3390/cancers7040875
APA StyleCallahan, B. P., & Wang, C. (2015). Hedgehog Cholesterolysis: Specialized Gatekeeper to Oncogenic Signaling. Cancers, 7(4), 2037-2053. https://doi.org/10.3390/cancers7040875