The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis
Abstract
:1. Introduction
2. L1 Family Members as Targets of Wnt/β-Catenin Signaling
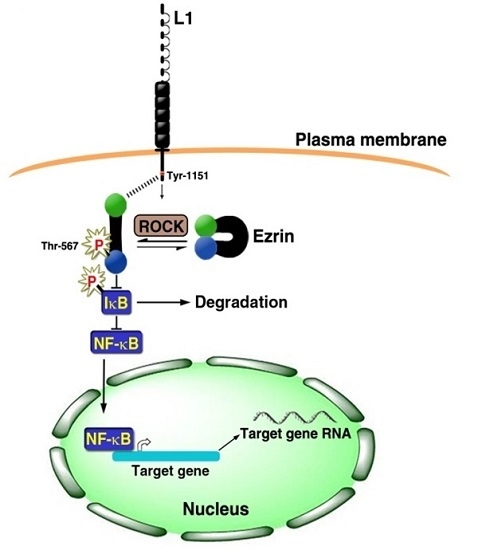
3. Signaling by L1 that Involves the NF-κB Pathway
4. Gene Expression Patterns of Human CRC Tissue and L1 Overexpressing CRC Cells
5. L1-Mediated Gene Expression and Intestinal Stem Cell Signature Genes
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| L1 | L1 cell adhesion molecule |
| APC | Adenomatous polyposis coli |
| IGFBP2 | Insulin-like growth factor binding protein 2 |
| SMOC2 | Secreted, modular, matricellular, Ca2+-binding protein 2 |
| NF-κB | Nuclear factor kappa B |
| IκB | Inhibitor of kappa B |
| EMT | Epithelial mesenchymal transition |
| MET | Mesenchymal epithelial transition |
| ILK | Integrin linked kinase |
| CRC | Colorectal cancer |
| ECM | Extracellular matrix |
| TCF/LEF | T cell factor/lymphoid enhancer factor |
| NrCAM | NgCAM-related neural cell adhesion molecule |
| NCAM | Neural cell adhesion molecule |
| ADAM10 | A disintegrin and metalloprotease 10 |
| Lgr5 | Leucine-rich repeat-containing G-coupled receptor 5 |
| Sp1 | Sephacryl phosphocellulose factor 1 |
References
- Lecuit, T.; Lenne, P.F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ze'ev, A.; Geiger, B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr. Opin. Cell Biol. 1998, 10, 629–639. [Google Scholar] [CrossRef]
- Heuberger, J.; Birchmeier, W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a002915. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.; von Kries, J.P.; Kuhl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996, 382, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Huber, O.; Korn, R.; McLaughlin, J.; Ohsugi, M.; Herrmann, B.G.; Kemler, R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech. Dev. 1996, 59, 3–10. [Google Scholar] [CrossRef]
- Molenaar, M.; van de Wetering, M.; Oosterwegel, M.; Peterson-Maduro, J.; Godsave, S.; Korinek, V.; Roose, J.; Destree, O.; Clevers, H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86, 391–399. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Cavallo, R.; Dooijes, D.; van Beest, M.; van Es, J.; Loureiro, J.; Ypma, A.; Hursh, D.; Jones, T.; Bejsovec, A.; et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 1997, 88, 789–799. [Google Scholar] [CrossRef]
- Wodarz, A.; Nusse, R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998, 14, 59–88. [Google Scholar] [CrossRef] [PubMed]
- Conacci-Sorrell, M.; Zhurinsky, J.; Ben-Ze'ev, A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. 2002, 109, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 547–553. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Ze'ev, A. Beta-catenin signaling in biological control and cancer. J. Cell Biochem. 2007, 102, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Moos, M.; Tacke, R.; Scherer, H.; Teplow, D.; Fruh, K.; Schachner, M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature 1988, 334, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Grumet, M. Cell adhesion molecules and their subgroups in the nervous system. Curr. Opin. Neurobiol. 1991, 1, 370–376. [Google Scholar] [CrossRef]
- Brummendorf, T.; Rathjen, F.G. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr. Opin. Neurobiol. 1996, 6, 584–593. [Google Scholar] [CrossRef]
- Lindner, J.; Rathjen, F.G.; Schachner, M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature 1983, 305, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Rathjen, F.G.; Schachner, M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984, 3, 1–10. [Google Scholar] [PubMed]
- Brummendorf, T.; Rathjen, F.G. Cell adhesion molecules 1: Immunoglobulin superfamily. Protein Profile 1995, 2, 963–1108. [Google Scholar] [PubMed]
- Jouet, M.; Rosenthal, A.; Armstrong, G.; MacFarlane, J.; Stevenson, R.; Paterson, J.; Metzenberg, A.; Ionasescu, V.; Temple, K.; Kenwrick, S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat. Genet. 1994, 7, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.V.; Kenwrick, S.; Willems, P.; Lemmon, V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995, 18, 168–172. [Google Scholar] [CrossRef]
- Fransen, E.; van Camp, G.; Vits, L.; Willems, P.J. L1-associated diseases: Clinical geneticists divide, molecular geneticists unite. Hum. Mol. Genet. 1997, 6, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Kamiguchi, H.; Hlavin, M.L.; Lemmon, V. Role of L1 in neural development: What the knockouts tell us. Mol. Cell. Neurosci. 1998, 12, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kenwrick, S.; Watkins, A.; de Angelis, E. Neural cell recognition molecule L1: Relating biological complexity to human disease mutations. Hum. Mol. Genet. 2000, 9, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Conacci-Sorrell, M.E.; Ben-Yedidia, T.; Shtutman, M.; Feinstein, E.; Einat, P.; Ben-Ze'ev, A. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002, 16, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Grumet, M.; Mauro, V.; Burgoon, M.P.; Edelman, G.M.; Cunningham, B.A. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J. Cell Biol. 1991, 113, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Ono, K.; Satoh, S.; Ishiguro, H.; Fujita, M.; Miwa, N.; Tanaka, T.; Tsunoda, T.; Yang, K.C.; Nakamura, Y.; et al. Identification of AF17 as a downstream gene of the beta-catenin/T-cell factor pathway and its involvement in colorectal carcinogenesis. Cancer Res. 2001, 61, 6345–6349. [Google Scholar] [PubMed]
- Gavert, N.; Conacci-Sorrell, M.; Gast, D.; Schneider, A.; Altevogt, P.; Brabletz, T.; Ben-Ze'ev, A. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J. Cell Biol. 2005, 168, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Sheffer, M.; Raveh, S.; Spaderna, S.; Shtutman, M.; Brabletz, T.; Barany, F.; Paty, P.; Notterman, D.; Domany, E.; et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007, 67, 7703–7712. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, V.; Farr, K.L.; Lagenaur, C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron 1989, 2, 1597–1603. [Google Scholar] [CrossRef]
- Haspel, J.; Grumet, M. The L1CAM extracellular region: A multi-domain protein with modular and cooperative binding modes. Front. Biosci. 2003, 8, s1210–s1225. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Levina, E.; Ohouo, P.; Baig, M.; Roninson, I.B. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006, 66, 11370–11380. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Mechtersheimer, S.; Gutwein, P.; Agmon-Levin, N.; Stoeck, A.; Oleszewski, M.; Riedle, S.; Postina, R.; Fahrenholz, F.; Fogel, M.; Lemmon, V.; et al. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol. 2001, 155, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Gast, D.; Joumaa, S.; Zentgraf, H.; Fogel, M.; Altevogt, D.P. ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J. 2003, 17, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Shmuel, A.; Lemmon, V.; Brabletz, T.; Ben-Ze'ev, A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J. Cell Sci. 2010, 123, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shmuel, A.; Shvab, A.; Gavert, N.; Brabletz, T.; Ben-Ze'ev, A. Global analysis of L1-transcriptomes identified IGFBP-2 as a target of ezrin and NF-kappaB signaling that promotes colon cancer progression. Oncogene 2013, 32, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- French, C.L.; Ye, F.; Revetta, F.; Zhang, B.; Coffey, R.J.; Washington, M.K.; Deane, N.G.; Beauchamp, R.D.; Weaver, A.M. Linking patient outcome to high throughput protein expression data identifies novel regulators of colorectal adenocarcinoma aggressiveness. F1000Res 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Shvab, A.; Sheffer, M.; Ben-Shmuel, A.; Haase, G.; Bakos, E.; Domany, E.; Ben-Ze'ev, A. c-Kit is suppressed in human colon cancer tissue and contributes to L1-mediated metastasis. Cancer Res. 2013, 73, 5754–5763. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, J.; Ronnstrand, L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radisky, D.C.; Yang, D.; Xu, R.; Radisky, E.S.; Bissell, M.J.; Bishop, J.M. MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat. Cell Biol. 2012, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Booth, C.; Pritchard, D.M. The intestinal epithelial stem cell: The mucosal governor. Int. J. Exp. Pathol. 1997, 78, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Moerer, P.; van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998, 19, 379–383. [Google Scholar] [PubMed]
- Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 2007, 27, 7551–7559. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Munoz, J.; Stange, D.E.; Schepers, A.G.; van de Wetering, M.; Koo, B.K.; Itzkovitz, S.; Volckmann, R.; Kung, K.S.; Koster, J.; Radulescu, S.; et al. The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent '+4' cell markers. EMBO J. 2012, 31, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Shvab, A.; Haase, G.; Ben-Shmuel, A.; Gavert, N.; Brabletz, T.; Dedhar, S.; Ben-Ze'ev, A. Induction of the intestinal stem cell signature gene SMOC-2 is required for L1-mediated colon cancer progression. Oncogene 2016, 35, 549–557. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haase, G.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis. Cancers 2016, 8, 48. https://doi.org/10.3390/cancers8050048
Haase G, Gavert N, Brabletz T, Ben-Ze’ev A. The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis. Cancers. 2016; 8(5):48. https://doi.org/10.3390/cancers8050048
Chicago/Turabian StyleHaase, Gal, Nancy Gavert, Thomas Brabletz, and Avri Ben-Ze’ev. 2016. "The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis" Cancers 8, no. 5: 48. https://doi.org/10.3390/cancers8050048
APA StyleHaase, G., Gavert, N., Brabletz, T., & Ben-Ze’ev, A. (2016). The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis. Cancers, 8(5), 48. https://doi.org/10.3390/cancers8050048