Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light
Abstract
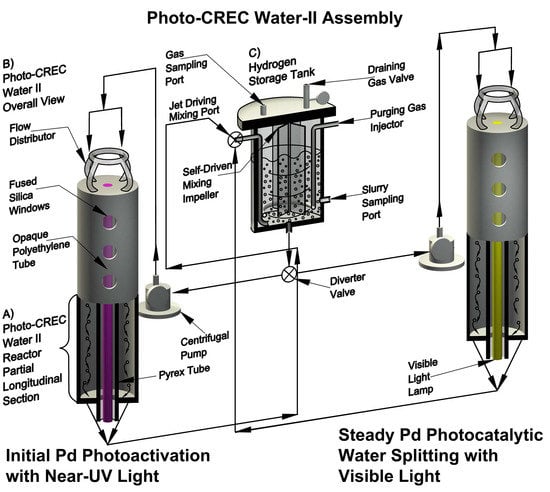
:1. Introduction
2. Results and Discussion
2.1. Photocatalyst Characterization
2.1.1. Adsorption-Desorption Isotherms
2.1.2. Hydrogen Chemisorption
2.1.3. X-ray Diffraction (XRD)
2.1.4. Temperature Programmed Reduction (TPR)
2.1.5. Band Gap
2.1.6. X-ray Photoelectron Spectroscopy (XPS)
2.2. Macroscopic Irradiation Energy Balance (MIEB)
2.3. Hydrogen Production
2.3.1. Precursor Near UV-Light Photoreduction
2.4. Quantum Yield (QY) Evaluation
Effect of Pd Addition on Quantum Yields
3. Experimental Methods
3.1. Photocatalyst Synthesis
3.2. Equipment
3.3. Photocatalyst Characterization
3.4. Hydrogen Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CO2 | Carbon dioxide |
| CH4 | Methane |
| C2H6 | Ethane |
| C2H4O | Acetaldehyde |
| c | Speed of light (3.0 × 108 m/s) |
| Dp | Pore diameter (cm) |
| e− | Electron |
| h+ | Hole |
| h | Planck’s constant (6.63 × 1034 J/s) |
| Ebg | Energy band gap (eV) |
| Eav | Average energy of a photon (kJ/mol photon) |
| F-127 | Poly (ethylene oxide)/poly (propylene oxide)/poly (ethylene oxide) |
| H• | Hydrogen radical |
| H2O | Water |
| I(λ) | Intensity of light (W/cm2) |
| OH− | Hydroxide ions |
| OH• | Hydroxide radicals |
| P-123 | Poly (ethylene glycol)-block-poly (propylene glycol)-block-poly (ethylene glycol) |
| P0 | Rate of photons emitted by the BLB lamp (einstein/s) |
| Pa | Rate of absorbed photons (einstein/s) |
| Pa-wall | Rate of photons absorbed by the inner pyrex glass (einstein/s) |
| Pbs | Rate of backscattered photons exiting the system (einstein/s) |
| Pd | Palladium |
| PdCl2 | Palladium II chloride |
| PEO | Poly (ethylene oxide) |
| Pfs | Rate of forward-scattered radiation (einstein/s) |
| Pi | Rate of photons reaching the reactor inner surface (einstein/s) |
| Pns | Rate of transmitted non-scattered radiation (einstein/s) |
| PPO | Poly (propylene oxide) |
| Pt | Rate of transmitted photons (einstein/s) |
| Pt | Platinum |
| q (θ, z, λ, t) | Net radiative flux over the lamp emission spectrum (μW/cm2) |
| t | Time (h) |
| TiO2 | Titanium dioxide |
| V | Total volume of the gas chamber (5716 cm3) |
| VP | pore volume |
| W | Weight (g) |
| Wt% | Weight percent (% m/m) |
| Greek symbols | |
| θ | Diffraction angle, also scattering angular angle (o) |
| λ | Wave length (nm) |
| φ | Quantum Yield Efficiency (%) |
| Acronyms | |
| Bg | Band Gap |
| BJH | Barrett–Joyner–Halenda Model |
| BLB | Black Light Blue Lamp |
| BET | Brunauer–Emmett–Teller Surface Area Method |
| CB | Conduction Band |
| DP25 | Degussa P25 (TiO2) |
| EISA | Evaporation-Induced-Self-Assembly |
| FID | Flame Ionization Detector |
| JCPDS | International Centre for Diffraction Data |
| K-M | Kubelka-Munk |
| MIEB | Macroscopic Irradiation Energy Balance |
| PCW-II | Photo-CREC Water II Reactor |
| PC | Photocatalyst Concentration |
| STP | Standard Temperature and Pressure (273 K and 1 atm) |
| TPR | Temperature Programmed Reduction |
| TCD | Thermal Conductivity Detector |
| UV | Ultraviolet |
| VB | Valence Band |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
Appendix A. Macroscopic Irradiation Energy Balance (MIEB)
- (a)
- Pi is the rate of photons reaching the slurry suspension:
- (b)
- Pbs represents the difference between Pi and Pt/c→0+. It is the rate of photons transmitted at a photocatalyst concentration approaching zero [28] as follows:
- (c)
- Pt accounts for the difference between the transmitted non-scattered radiation (Pns) and the forward-scattered radiation (Pfs):
Appendix B. Photocatalyst Synthesis—EISA Method

Appendix C. Lamp Characterization



Appendix D. Quantum Yield Calculation
References
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Highly Stable Water Splitting on Oxynitride TaON Photoanode System under Visible Light Irradiation. J. Am. Chem. Soc. 2012, 134, 6968–6971. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfide Sm2Ti2S2O5 as a Stable Photocatalyst for Water Oxidation and Reduction under Visible Light Irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 2002, 124, 13547–13553. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pierre, A.; Kibria, M.G.; Cui, K.; Han, X.; Bevan, K.H.; Guo, H.; Paradis, S.; Hakima, A.-R.; Mi, Z. Wafer-Level Photocatalytic Water Splitting on GaN Nanowire Arrays Grown by Molecular Beam Epitaxy. Nano Lett. 2011, 11, 2353–2357. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Sugihara, H. Development of New Photocatalytic Water Splitting into H2 and O2 using Two Different Semiconductor Photocatalysts and a Shuttle Redox Mediator IO3-/I-. J. Phys. Chem. B 2005, 109, 16052–16061. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-Doped Ti 3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ipsakis, D.; Voutetakis, S.; Seferlis, P.; Stergiopoulos, F.; Elmasides, C. Power management strategies for a stand-alone power system using renewable energy sources and hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 7081–7095. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q.M. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Mills, A. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Abe, R. Significant effect of iodide addition on water splitting into H2 and O2 over Pt-loaded TiO2 photocatalyst: Suppression of backward reaction. Chem. Phys. Lett. 2003, 371, 360–364. [Google Scholar] [CrossRef]
- Mills, A. Photosensitised dissociation of water using dispersed suspensions of n-type semiconductors. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1982, 12, 3659–3669. [Google Scholar] [CrossRef]
- López, C.R.; Melián, E.P.; Ortega Méndez, J.A.; Santiago, D.E.; Rodríguez, J.M.; González Díaz, O. Comparative study of alcohols as sacrificial agents in H2production by heterogeneous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Galińska, A. Photocatalytic Water Splitting over Pt−TiO2 in the Presence of Sacrificial Reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Acar, C. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Melián, E.P.; López, C.R.; Méndez, A.O.; Díaz, O.G.; Suárez, M.N.; Rodríguez, J.M.D.; Navío, J.A.; Heviaac, D.F. Hydrogen production using Pt-loaded TiO2 photocatalysts. Int. J. Hydrogen Energy 2013, 38, 11737–11748. [Google Scholar] [CrossRef]
- Olivo, A.; Ghedini, E.; Signoretto, M.; Compagnoni, M.; Rossetti, I. Liquid vs. Gas Phase CO2 Photoreduction Process: Which Is the Effect of the Reaction Medium? Energies 2017, 10, 1394. [Google Scholar] [CrossRef] [Green Version]
- Castro, A.L. Synthesis of anatase TiO2 nanoparticles with high temperature stability and photocatalytic activity. Solid State Sci. 2008, 10, 602–606. [Google Scholar] [CrossRef]
- Holloway, P.; McGuire, G. Handbook of Compound Semiconductor; Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Yang, J. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, M.; Guan, Z.; Li, Q.; Yang, J. Effect of platinum dispersion on photocatalytic performance of Pt-TiO2. J. Nanopart. Res. 2018, 20, 60. [Google Scholar] [CrossRef]
- Escobedo, S.; Serrano, B.; Calzada, A.; Moreira, J.; De Lasa, H. Hydrogen production using a platinum modified TiO2 photocatalyst and an organic scavenger. Kinetic modeling. Fuel 2016, 181, 438–449. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valadés-Pelayo, P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, M.Y.; Mohamed, R.M. Novel Pd/TiO2 nanocomposite prepared by modified sol–gel method for photocatalytic degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 2013, 576, 201–207. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Kuncaka, A.; Sutarno, S. Application of Photocatalytic Reduction Method with TiO2 for Gold Recovery. Chemistry 2015, 3, 207–211. [Google Scholar]
- Lasa, H.D.; Rosales, B.S.; Moreira, J.; Valades‐Pelayo, P. Efficiency Factors in Photocatalytic Reactors: Quantum Yield and Photochemical Thermodynamic Efficiency Factor. Chem. Eng. Technol. 2016, 39, 51–65. [Google Scholar] [CrossRef]
- Endang, H.A.; Nurul, T.W. Photoreduction Processes over TiO2 Photocatalyst. In Photocatalysts—Applications and Attributes; IntechOpen: London, UK, 2018; p. 17. [Google Scholar]
- Pan, X.; Xu, Y.J. Defect-mediated growth of noble-metal (Ag, Pt, and Pd) nanoparticles on TiO2 with oxygen vacancies for photocatalytic redox reactions under visible light. J. Phys. Chem. C 2013, 117, 17996–18005. [Google Scholar] [CrossRef]
- AutoChem 2920 Automated Catalyst Characterization System. In Operator’s Manual; Micromeritics Instrument Corporation: Norcross, GA, USA, 2014.
- Treacy, J.P.W.; Hussain, H.; Torrelles, X.; Grinter, D.; Cabailh, G.; Bikondoa, O.; Nicklin, C.; Selcuk, S.; Selloni, A.; Lindsay, R.; et al. Geometric structure of anatase TiO2(101). Phys. Rev. B 2017, 95, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, V.G.; Owen, S.L.; Abrokwah, R.Y.; Kuila, D. Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: Effect of metal-support interactions on steam reforming of methanol. J. Mol. Catal. A Chem. 2015, 408, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Mendez, C.M.; Olivero, H.; Damiani, D.E.; Volpe, M.A. On the role of Pd β-hydride in the reduction of nitrate over Pd based catalyst. Appl. Catal. B Environ. 2008, 84, 156–161. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd° oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607. [Google Scholar] [CrossRef]
- Bratan, V.; Munteanu, C.; Hornoiu, C.; Vasile, A.; Papa, F.; State, R.; Preda, S.; Culita, D.; Ionescu, N.I. CO oxidation over Pd supported catalysts—In situ study of the electric and catalytic properties. Appl. Catal. B Environ. 2017, 207, 166–173. [Google Scholar] [CrossRef]
- González, C.A.; Ardila, A.N.; de Correa, C.M.; Martínez, M.A.; Fuentes-Zurita, G. Pd/TiO2 Washcoated Cordierite Minimonoliths for Hydrodechlorination of Light Organochlorinated Compounds. Ind. Eng. Chem. Res. 2007, 46, 7961–7969. [Google Scholar] [CrossRef]
- Tauc, A.; Grigorovici, J.; Vancu, R. Optical Properties and Electronic Structure of Amorphous Germanium. Basic Solid State Phys. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Santara, B.; Pal, B.; Giri, P.K. Signature of strong ferromagnetism and optical properties of Co doped TiO2 nanoparticles. J. Appl. Phys. 2011, 110, 114322. [Google Scholar] [CrossRef]
- Khairy, W.; Zakaria, M. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Ola, O.; Maroto-Valer, M.M. Transition metal oxide based TiO2 nanoparticles for visible light induced CO2 photoreduction. Appl. Catal. A Gen. 2015, 502, 114–121. [Google Scholar] [CrossRef]
- Sobana, N.; Muruganadham, M.; Swaminathan, M. Nano-Ag particles doped TiO2 for efficient photodegradation of Direct azo dyes. J. Mol. Catal. A Chem. 2006, 258, 124–132. [Google Scholar] [CrossRef]
- Leong, K.H.; Chu, H.Y.; Ibrahim, S.; Saravanan, P. Palladium nanoparticles anchored to anatase TiO2 for enhanced surface plasmon resonance-stimulated, visible-light-driven photocatalytic activity. Beilstein J. Nanotechnol. 2015, 6, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ma, F.; Li, K.; Guo, Y.; Hu, J.; Li, W.; Huo, M.; Guo, Y. Mixed phase titania nanocomposite codoped with metallic silver and vanadium oxide: New efficient photocatalyst for dye degradation. J. Hazard. Mater. 2010, 175, 429–438. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Nitrogen / Palladium-Codoped TiO2 for Efficient Visible Light Photocatalytic Dye Degradation. J. Phys. Chem. C 2011, 115, 22110–22120. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Park, Y.; Kim, J.; Ryu, W.H.; Rho, J.; Kim, K. Photodeposited metal-semiconductor nanocomposites and their applications. J. Mater. 2018, 4, 83–94. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S. Performance and characterization of supported metal catalysts for complete oxidation of formaldehyde at low temperatures. Appl. Catal. B Environ. 2007, 73, 282–291. [Google Scholar] [CrossRef]
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Diak, M.; Emília Quinta-Ferreira, M.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Photocatalytic ozonation using doped TiO2 catalysts for the removal of parabens in water. Sci. Total Environ. 2017, 609, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Maicu, M.; Hidalgo, M.C.; Colón, G.; Navío, J.A. Comparative study of the photodeposition of Pt, Au and Pd on pre-sulphated TiO2 for the photocatalytic decomposition of phenol. J. Photochem. Photobiol. A Chem. 2011, 217, 275–283. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Morgan, D.J.; Morton, C.A.; Egerton, T.A.; Kennedy, J.; Jones, W. Rutile TiO2–Pd Photocatalysts for Hydrogen Gas Production from Methanol Reforming. Top. Catal. 2015, 58, 70–76. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photocatalytic hydrogen production under near-UV using Pd-doped mesoporous TiO2 and ethanol as organic scavenger. Catalysts 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Riyapan, S.; Boonyongmaneerat, Y.; Mekasuwandumrong, O.; Yoshida, H.; Fujita, S.-I.; Arai, M.; Panpranot, J. Improved catalytic performance of Pd/TiO2 in the selective hydrogenation of acetylene by using H2-treated sol-gel TiO2. J. Mol. Catal. A Chem. 2014, 383–384, 182–187. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tonbul, Y.; Özkar, S. Nanoceria supported palladium (0) nanoparticles: Superb catalyst in dehydrogenation of formic acid at room temperature. Appl. Catal. B Environ. 2017, 206, 384–392. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Onderwaater, W.G.; Taranovskyy, A.; van Baarle, G.C.; Frenken, J.W.M.; Groot, I.M.N. In Situ Optical Reflectance Difference Observations of CO Oxidation over Pd (100). J. Phys. Chem. C 2017, 121, 11407–11415. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, T.N.; Vaz, M.D.O.; Ramakrishnappa, T.; Teixeira, S.R.; Dupont, J.; Pai, R.K.; Banuprakash, G. The heterojunction effect of Pd on TiO2 for visible light photocatalytic hydrogen generation via water splitting reaction and photodecolorization of trypan blue dye. J. Mater. Sci. Mater. Electron. 2018, 29, 11132–11143. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Fernández-García, M.; Kubacka, A. Bimetallic Pt-Pd co-catalyst Nb-doped TiO2 materials for H2 photo-production under UV and Visible light illumination. Appl. Catal. B Environ. 2018, 238, 533–545. [Google Scholar] [CrossRef]
- Sokolov, S.; Ortel, E.; Kraehnert, R. Mesoporous titania films with adjustable pore size coated on stainless steel substrates. Mater. Res. Bull. 2009, 44, 2222–2227. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Bhaumik, A. Self-assembled TiO2 nanoparticles: Mesoporosity, optical and catalytic properties. Dalt. Trans. 2010, 39, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Syu, H.-R. One-step fabrication of N-doped mesoporous TiO2 nanoparticles by self-assembly for photocatalytic water splitting under visible light. Appl. Energy 2012, 100, 148–154. [Google Scholar] [CrossRef]
- Yu, J.C.; Wang, X.; Fu, X. Pore-Wall Chemistry and Photocatalytic Activity of Mesoporous Titania Molecular Sieve Films. Chem. Mater. 2004, 16, 1523–1530. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Li, H.; Zou, C.; Wang, H.; Li, D. Mesoporous TiO2 thin films exhibiting enhanced thermal stability and controllable pore size: Preparation and photocatalyzed destruction of Cationic Dyes. ACS Appl. Mater. Interfaces 2014, 6, 1623–1631. [Google Scholar] [CrossRef]
- Salas, S.E.; de Lasa, H. Photocatalytic Water Splitting using a Modified Pt-TiO2. Kinetic Modeling and Hydrogen Production Efficiency. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 27 August 2013. [Google Scholar]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reaction Engineering; Springer Science: New York, NY, USA, 2005. [Google Scholar]
- Fagerlund, G. Determination of specific surface by the BET method. Matér. Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Slav, A. Optical characterization of TiO2-Ge nanocomposite films obtained by reactive magnetron sputtering. Dig. J. Nanomater. Biostruct. 2011, 6, 915–920. [Google Scholar]
- Guo, S.P.; Li, J.C.; Xu, Q.T.; Ma, Z.; Xue, H.G. Recent achievements on polyanion-type compounds for sodium-ion batteries: Syntheses, crystal chemistry and electrochemical performance. J. Power Sources 2017, 361, 285–299. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Lisowski, P.; Łomot, D.; Chernyayeva, O.; Lisovytskiy, D. Sonophotodeposition of Bimetallic Photocatalysts Pd-Au/TiO2: Application to Selective Oxidation of Methanol to Methyl Formate. ChemSusChem 2015, 8, 1676–1685. [Google Scholar] [CrossRef]
- Sanchez, C.; Boissière, C.; Grosso, D.; Laberty, C.; Nicole, L. Design, Synthesis, and Properties of Inorganic and Hybrid Thin Films Having Periodically Organized Nanoporosity. Chem. Mater. 2008, 20, 682–737. [Google Scholar] [CrossRef]
- Ortel, E.; Polte, J.; Bernsmeier, D.; Eckhardt, B.; Paul, B.; Bergmann, A.; Strasser, P.; Emmerling, F.; Kraehnert, R. Pd/TiO2 coatings with template-controlled mesopore structure as highly active hydrogenation catalyst. Appl. Catal. A Gen. 2015, 493, 25–32. [Google Scholar] [CrossRef]
- Salaices, M.; Serrano, B.; de Lasa, H. Photocatalytic Conversion of Organic Pollutants Extinction Coefficients and Quantum Efficiencies. Ind. Eng. Chem. Res. 2001, 40, 5455–5464. [Google Scholar] [CrossRef]













| Photocatalyst | SBET (m2 g−1) | DpBJH (4 VpBJH/SBET) (nm) | VpBJH (cm3g−1) |
|---|---|---|---|
| DP-25 | 59 | 7.5 | 0.11 |
| TiO2 | 140 | 17.5 | 0.61 |
| 0.25 wt% Pd–TiO2 | 131 | 16.5 | 0.53 |
| 0.50 wt% Pd–TiO2 | 124 | 16.8 | 0.52 |
| 1.00 wt% Pd–TiO2 | 123 | 21.2 | 0.65 |
| 2.50 wt% Pd–TiO2 | 122 | 19.9 | 0.60 |
| 5.00 wt% Pd–TiO2 | 119 | 18.9 | 0.56 |
| Photocatalyst | Metal Dispersion (%) |
|---|---|
| 0.25 wt% Pd–TiO2 | 75 |
| 0.50 wt% Pd–TiO2 | 27 |
| 1.00 wt% Pd–TiO2 | 26 |
| 2.50 wt% Pd–TiO2 | 12 |
| 5.00 wt% Pd–TiO2 | 8 |
| Photocatalyst | Crystallite Size (nm) |
|---|---|
| DP 25 | 21 |
| TiO2 | 9 |
| 0.25 wt% Pd -TiO2 | 11 |
| 0.50 wt% Pd -TiO2 | 11 |
| 1.00 wt% Pd -TiO2 | 11 |
| 2.50 wt% Pd -TiO2 | 13 |
| 5.00 wt% Pd -TiO2 | 14 |
| Photocatalyst | Band Gap (eV) |
|---|---|
| DP-25 | 3.10 |
| TiO2 | 2.99 |
| 0.25 wt% Pd–TiO2 | 2.51 |
| 0.50 wt% Pd–TiO2 | 2.55 |
| 1.00 wt% Pd–TiO2 | 2.60 |
| 2.50 wt% Pd–TiO2 | 2.67 |
| 5.00 wt% Pd–TiO2 | 2.67 |
| Peak Name | Before Photoreduction | After 60 min of Photoreduction Using Near-UV Irradiation | ||||
|---|---|---|---|---|---|---|
| Binding Energy | FWHM | % Area | Pos | FWHM | % Area | |
| Pd 3d3/2 PdO | 341.54 | 2.00 | 50.2 | 341.49 | 2.00 | 18.3 |
| Pd 3d3/2 Pd° | 339.69 | 1.13 | 49.8 | 339.56 | 1.29 | 81.7 |
| Pd 3d5/2 PdO | 336.28 | 2.00 | 50.2 | 336.23 | 2.00 | 18.3 |
| Pd 3d5/2 Pd° | 334.43 | 1.13 | 49.8 | 334.30 | 1.29 | 81.7 |
| Catalyst Loading | Pa (Einstein/s) |
|---|---|
| TiO2 | 2.23 × 10−6 |
| 0.25 wt% Pd - TiO2 | 4.37 × 10−6 |
| 0.50 wt% Pd- TiO2 | 4.45 × 10−6 |
| 1.00 wt% Pd- TiO2 | 5.62 × 10−6 |
| 2.50 wt% Pd- TiO2 | 4.87 × 10−6 |
| 5.00 wt% Pd- TiO2 | 4.81 × 10−6 |
| Photocatalyst | QY (%) (a) | QY (%) (b) |
|---|---|---|
| TiO2 | 0.23 | - |
| 0.25 wt% Pd TiO2 | 1.13 | 1.58 |
| 0.50 wt% Pd TiO2 | 0.34 | 1.07 |
| 1.00 wt% Pd TiO2 | 0.30 | 0.80 |
| 2.50 wt% Pd TiO2 | 0.10 | 0.79 |
| 5.00 wt% Pd TiO2 | 0.10 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinque, B.; Escobedo, S.; de Lasa, H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts 2020, 10, 74. https://doi.org/10.3390/catal10010074
Rusinque B, Escobedo S, de Lasa H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts. 2020; 10(1):74. https://doi.org/10.3390/catal10010074
Chicago/Turabian StyleRusinque, Bianca, Salvador Escobedo, and Hugo de Lasa. 2020. "Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light" Catalysts 10, no. 1: 74. https://doi.org/10.3390/catal10010074
APA StyleRusinque, B., Escobedo, S., & de Lasa, H. (2020). Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts, 10(1), 74. https://doi.org/10.3390/catal10010074