A Selective Synthesis of TaON Nanoparticles and Their Comparative Study of Photoelectrochemical Properties
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Photocatalyst
2.1.1. Analysis of Nanoparticle Morphology Using Scanning Electron Microscopy (SEM)
2.1.2. HRTEM Analysis of the Photocatalysts Samples before and after Nitridation
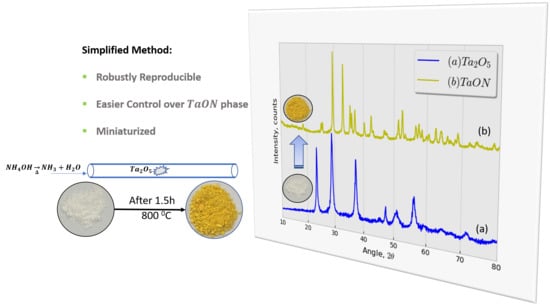
2.1.3. Phase Identification Using X-ray Diffraction (XRD)
2.1.4. Optical Analysis Using Spectroscopy
2.2. Evaluation of the Photocatalytic Activity towards Methylene Blue Degradation
2.2.1. Methylene Blue Absorbance and Photolytic Conversion
2.2.2. Comparing Photoactivity of In-House Ta2O5, TaON, and Ta3N5
3. Discussion
3.1. Chronoamperometry (j/t) and Linear Sweep Voltammetry (j/V) Measurements
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. Evaluation of the Reusability of the Photocatalysts
3.4. Methylene Blue Oxidation Routes
4. Experimental
4.1. Synthesis Approach
4.2. Material Characterization
4.3. Determination of Photocatalytic Properties
4.4. Determination of Photoelectrochemical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Dolat, D.; Mozia, S.; Ohtani, B.; Morawski, A.W. Nitrogen, iron-single modified (N-TiO2, Fe-TiO2) and co-modified (Fe,N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Li, F.; Zhang, Y.; Fu, X.; Xu, Y.-J. Composites of Titanate Nanotube and Carbon Nanotube as Photocatalyst with High Mineralization Ratio for Gas-Phase Degradation of Volatile Aromatic Pollutant. J. Phys. Chem. C 2011, 115, 7880–7886. [Google Scholar] [CrossRef]
- Khanal, V.; Ragsdale, W.; Gupta, S.; Subramanian, V.R. Insights into the photoactivity of iron modified bismuth titanate (Fe_BTO) nanoparticles. Catal. Today 2018, 300, 81–88. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467. [Google Scholar] [CrossRef]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 Core–Shell Nanostructures for Enhanced Photoelectrochemical Water Splitting under Solar Light Illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef]
- Naveenraj, S.; Lee, G.-J.; Anandan, S.; Wu, J.J. Nanosized tantala based materials—Synthesis and applications. Mater. Res. Bull. 2015, 67, 20–46. [Google Scholar] [CrossRef]
- Nikishina, E.E.; Lebedeva, E.N.; Drobot, D.V. Niobium- and tantalum-containing oxide materials: Synthesis, properties, and application. Inorg. Mater. 2012, 48, 1243–1260. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Li, H.; Li, C.; Luo, Z.; Gong, J. Transparent Ta2O5 Protective Layer for Stable Silicon Photocathode under Full Solar Spectrum. Ind. Eng. Chem. Res. 2019, 58, 5510–5515. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Z.; Dou, M.; Wang, F. Highly Dispersed and Crystalline Ta2O5 Anchored Pt Electrocatalyst with Improved Activity and Durability Toward Oxygen Reduction: Promotion by Atomic-Scale Pt-Ta2O5 Interactions. ACS Catal. 2019, 9, 3278–3288. [Google Scholar] [CrossRef]
- Fu, W.; Zhuang, P.; Chee, M.O.; Dong, P.; Ye, M.; Shen, J. Oxygen Vacancies in Ta2O5 Nanorods for Highly Efficient Electrocatalytic N2 Reduction to NH3 under Ambient Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9622–9628. [Google Scholar] [CrossRef]
- Dang, H.X.; Hahn, N.T.; Park, H.S.; Bard, A.J.; Mullins, C.B. Nanostructured Ta3N5 Films as Visible-Light Active Photoanodes for Water Oxidation. J. Phys. Chem. C 2012, 116, 19225–19232. [Google Scholar] [CrossRef]
- Ho, C.-T.; Low, K.-B.; Jash, P.; Shen, H.; Snee, P.T.; Meyer, R.J. Formation of Sol–Gel-Derived TaOxNy Photocatalysts. Chem. Mater. 2011, 23, 4721–4725. [Google Scholar] [CrossRef]
- Harb, M.; Sautet, P.; Nurlaela, E.; Raybaud, P.; Cavallo, L.; Domen, K.; Basset, J.M.; Takanabe, K. Tuning the properties of visible-light-responsive tantalum (oxy)nitride photocatalysts by non-stoichiometric compositions: A first-principles viewpoint. Phys. Chem. Chem. Phys. 2014, 16, 20548–20560. [Google Scholar] [CrossRef]
- Oi-Uchisawa, J.; Obuchi, A.; Enomoto, R.; Liu, S.; Nanba, T.; Kushiyama, S. Catalytic performance of Pt supported on various metal oxides in the oxidation of carbon black. Appl. Catal. B Environ. 2000, 26, 17–24. [Google Scholar] [CrossRef]
- Gan, J.; Lu, X.; Tong, Y. Towards highly efficient photoanodes: Boosting sunlight-driven semiconductor nanomaterials for water oxidation. Nanoscale 2014, 6, 7142. [Google Scholar] [CrossRef]
- Vo, V.; Van Kim, N.; Nga, N.T.; Trung, N.T.; Van Hanh, P.; Hoang, L.H.; Kim, S.J. Preparation of g-C3N4/Ta2O5 Composites with Enhanced Visible-Light Photocatalytic Activity. J. Electron. Mater. 2016, 45, 2334–2340. [Google Scholar] [CrossRef]
- Ghugal, S.G.; Umare, S.S.; Sasikala, R. Photocatalytic mineralization of anionic dyes using bismuth doped CdS–Ta2O5 composite. RSC Adv. 2015, 5, 63393–63400. [Google Scholar] [CrossRef]
- Tao, C.; Xu, L.; Guan, J. Well-dispersed mesoporous Ta2O5 submicrospheres: Enhanced photocatalytic activity by tuning heating rate at calcination. Chem. Eng. J. 2013, 229, 371–377. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Domen, K.; Takanabe, K. Photocatalytic hydrogen production using visible-light-responsive Ta 3N5 photocatalyst supported on monodisperse spherical SiO2 particulates. Mater. Res. Bull. 2014, 49, 58–65. [Google Scholar] [CrossRef]
- Takahara, Y.; Kondo, J.N.; Takata, T.; Lu, D.; Domen, K. Mesoporous Tantalum Oxide. 1. Characterization and Photocatalytic Activity for the Overall Water Decomposition. Chem. Mater. 2001, 13, 1194–1199. [Google Scholar] [CrossRef]
- Grewe, T.; Tüysüz, H. Alkali metals incorporated ordered mesoporous tantalum oxide with enhanced photocatalytic activity for water splitting. J. Mater. Chem. A 2016, 4, 3007–3017. [Google Scholar] [CrossRef]
- Xu, L.; Guan, J.; Shi, W. Enhanced Interfacial Charge Transfer and Visible Photocatalytic Activity for Hydrogen Evolution from a Ta2O5 -based Mesoporous Composite by the Incorporation of Quantum-Sized CdS. ChemCatChem 2012, 4, 1353–1359. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Migowski, P.; Wender, H.; Eberhardt, D.; Weibel, D.E.; Sonaglio, F.C.; Zapata, M.J.; Dupont, J.; Feil, A.F.; Teixeira, S.R. Ta2O5 Nanotubes Obtained by Anodization: Effect of Thermal Treatment on the Photocatalytic Activity for Hydrogen Production. J. Phys. Chem. C 2012, 116, 14022–14030. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Tang, X.; Luo, Y.; Zeng, Q.; Deng, X.; Ding, S.; Sun, Y. Gold nanoparticles embedded in Ta 2O5/Ta3N5 as active visible-light plasmonic photocatalysts for solar hydrogen evolution. J. Mater. Chem. A 2014, 2, 14927–14939. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Chen, C.; Hu, X.; Zhao, J.; Yu, J.C. Fe3+/Fe2+ cycling promoted by Ta3N5 under visible irradiation in Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2007, 75, 256–263. [Google Scholar] [CrossRef]
- Rooke, J.C.; Barakat, T.; Brunet, J.; Li, Y.; Finol, M.F.; Lamonier, J.F.; Giraudon, J.M.; Cousin, R.; Siffert, S.; Su, B.L. Hierarchically nanostructured porous group Vb metal oxides from alkoxide precursors and their role in the catalytic remediation of VOCs. Appl. Catal. B Environ. 2015, 162, 300–309. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Liu, S.; Wang, S. A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl. Catal. B Environ. 2014, 146, 162–168. [Google Scholar] [CrossRef]
- Ma, S.S.K.; Maeda, K.; Hisatomi, T.; Tabata, M.; Kudo, A.; Domen, K. A Redox-Mediator-Free Solar-Driven Z-Scheme Water-Splitting System Consisting of Modified Ta3N5 as an Oxygen-Evolution Photocatalyst. Chem. A. Eur. J. 2013, 19, 7480–7486. [Google Scholar] [CrossRef]
- Ma, S.S.K.; Hisatomi, T.; Maeda, K.; Moriya, Y.; Domen, K. Enhanced Water Oxidation on Ta3N5 Photocatalysts by Modification with Alkaline Metal Salts. J. Am. Chem. Soc. 2012, 134, 19993–19996. [Google Scholar] [CrossRef]
- Higashi, T.; Nishiyama, H.; Suzuki, Y.; Sasaki, Y.; Hisatomi, T.; Katayama, M.; Minegishi, T.; Seki, K.; Yamada, T.; Domen, K. Transparent Ta3N5 Photoanodes for Efficient Oxygen Evolution toward the Development of Tandem Cells. Angew. Chem. Int. Ed. 2019, 58, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Morikawa, T.; Saeki, S.; Kajino, T.; Motohiro, T. Visible-Light-Induced Selective CO2 Reduction Utilizing a Ruthenium Complex Electrocatalyst Linked to a p-Type Nitrogen-Doped Ta2O5 Semiconductor. Angew. Chem. Int. Ed. 2010, 49, 5101–5105. [Google Scholar] [CrossRef]
- Huang, J.; Ma, R.; Ebina, Y.; Fukuda, K.; Takada, K.; Sasaki, T. Layer-by-Layer Assembly of TaO3 Nanosheet/Polycation Composite Nanostructures: Multilayer Film, Hollow Sphere, and Its Photocatalytic Activity for Hydrogen Evolution. Chem. Mater. 2010, 22, 2582–2587. [Google Scholar] [CrossRef]
- Li, J.; Dai, W.; Wu, G.; Guan, N.; Li, L. Fabrication of Ta2O5 films on tantalum substrate for efficient photocatalysis. Catal. Commun. 2015, 65, 24–29. [Google Scholar] [CrossRef]
- Yokomichi, Y.; Nakayama, T.; Okada, O.; Yokoi, Y.; Takahashi, I.; Uchida, H.; Ishikawa, H.; Yamaguchi, R.; Matsui, H.; Yamabe, T. CATALYSIS TODAY i Fundamental study on the NO x direct decomposition catalysts. Catal. Today 1996, 29, 155–160. [Google Scholar] [CrossRef]
- Subramanian, V.; Kamat, P.V.; Wolf, E.E. Mass-transfer and kinetic studies during the photocatalytic degradation of an azo dye on optically transparent electrode thin film. Ind. Eng. Chem. Res. 2003, 42, 2131–2138. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, F.; Man, Y.; Tian, Q.; He, Y.; Wu, N. Preparation and performances of nanosized Ta2O5 powder photocatalyst. J. Solid State Chem. 2005, 178, 224–229. [Google Scholar] [CrossRef]
- Murase, T.; Irie, H.; Hashimoto, K. Visible light sensitive photocatalysts, nitrogen-doped Ta2O5 powders. J. Phys. Chem. B 2004, 108, 15803–15807. [Google Scholar] [CrossRef]
- Ndiege, N.; Wilhoite, T.; Subramanian, V.; Shannon, M.A.; Masel, R.I. Sol-gel synthesis of thick Ta2O5 films. Chem. Mater. 2007, 19, 3155–3161. [Google Scholar] [CrossRef]
- Wang, S.; Guan, Y.; Wang, L.; Zhao, W.; He, H.; Xiao, J.; Yang, S.; Sun, C. Fabrication of a novel bifunctional material of BiOI/Ag3VO4 with high adsorption–photocatalysis for efficient treatment of dye wastewater. Appl. Catal. B Environ. 2015, 168, 448–457. [Google Scholar] [CrossRef]
- Jones, D.R.; Gomez, V.; Bear, J.C.; Rome, B.; Mazzali, F.; McGettrick, J.D.; Lewis, A.R.; Margadonna, S.; Al-Masry, W.A.; Dunnill, C.W. Active removal of waste dye pollutants using Ta3N5/W18O49 nanocomposite fibres. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garza-Tovar, L.L.; Torres-Martínez, L.M.; Rodríguez, D.B.; Gómez, R.; del Angel, G. Photocatalytic degradation of methylene blue on Bi2MNbO7 (M = Al, Fe, In, Sm) sol–gel catalysts. J. Mol. Catal. A Chem. 2006, 247, 283–290. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Kim, E.S.; Nishimura, N.; Magesh, G.; Kim, J.Y.; Jang, J.W.; Jun, H.; Kubota, J.; Domen, K.; Lee, J.S. Fabrication of CaFe2O4/TaON heterojunction photoanode for photoelectrochemical water oxidation. J. Am. Chem. Soc. 2013, 135, 5375–5383. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Sahu, M.; Hussain, S. Under dark and visible light: Fast degradation of methylene blue in the presence of Ag-In-Ni-S nanocomposites. J. Mater. Chem. A 2015, 3, 15616–15625. [Google Scholar] [CrossRef] [Green Version]
- Smith, Y.R.; Kar, A.; Subramanian, V. Investigation of physicochemical parameters that influence photocatalytic degradation of methyl orange over TIO2 nanotubes. Ind. Eng. Chem. Res. 2009, 48, 10268–10276. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Smith, Y.R.; Misra, M.; Ravi, V. Subramanian, Electrochemically assisted photocatalytic degradation of methyl orange using anodized titanium dioxide nanotubes. Appl. Catal. B Environ. 2008, 84, 372–378. [Google Scholar] [CrossRef]
- Mukherjee, B.; Wilson, W.; Ravi, V. Subramanian, TiO2 nanotube (T_NT) surface treatment revisited: Implications of ZnO, TiCl4, and H2O2 treatment on the photoelectrochemical properties of T_NT and T_NT–CdSe. Nanoscale 2013, 5, 269–274. [Google Scholar] [CrossRef]
- Mukherjee, B.; Peterson, A.; Subramanian, V. 1D CdS/PbS heterostructured nanowire synthesis using cation exchange. Chem. Commun. 2012, 48, 2415. [Google Scholar] [CrossRef]
- Gupta, S.; de Leon, L.; Ravi, V. Subramanian, Mn-modified Bi2 Ti2O7 photocatalysts: Bandgap engineered multifunctional photocatalysts for hydrogen generation. Phys. Chem. Chem. Phys. 2014, 16, 12719–12727. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Faisal, M.; Harraz, F.A.; Al-Hajry, A.; Al-Sehemi, A.G. Synthesis of mesoporous sulfur-doped Ta2O5nanocomposites and their photocatalytic activities. J. Colloid Interface Sci. 2016, 471, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Huang, J. Preparation of mesoporous tantalum oxide and its enhanced photocatalytic activity. Mater. Lett. 2011, 65, 64–66. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Li, C.; Liang, H.C. Structures and photocatalytic behavior of tantalum-oxynitride thin films. Thin Solid Films 2011, 519, 4699–4704. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Lee, S.-Y.; Chang, K.-S. Facile Fabrication of a Photocatalyst of Ta4N5 Nanocolumn Arrays by Using Reactive Sputtering. J. Electrochem. Soc. 2015, 162, H371–H375. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Liu, S.; Wang, S. Graphene facilitated visible light photodegradation of methylene blue over titanium dioxide photocatalysts. Chem. Eng. J. 2013, 214, 298–303. [Google Scholar] [CrossRef]
- Jiang, F.; Yan, T.; Chen, H.; Sun, A.; Xu, C.; Wang, X. A g-C3N4 -CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation. Appl. Surf. Sci. 2014, 295, 164–172. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Cui, H.; Zhao, W.; Zhang, H.; Huang, F. Gray Ta2O5 Nanowires with Greatly Enhanced Photocatalytic Performance. ACS Appl. Mater. Interfaces 2016, 8, 122–127. [Google Scholar] [CrossRef]
- Ji, K.; Deng, J.; Zang, H.; Han, J.; Arandiyan, H.; Dai, H. Fabrication and high photocatalytic performance of noble metal nanoparticles supported on 3DOM InVO4-BiVO4 for the visible-light-driven degradation of rhodamine B and methylene blue. Appl. Catal. B Environ. 2015, 165, 285–295. [Google Scholar] [CrossRef]
- Umrao, S.; Abraham, S.; Theil, F.; Pandey, S.; Ciobota, V.; Shukla, P.K.; Rupp, C.J.; Chakraborty, S.; Ahuja, R.; Popp, J.; et al. A possible mechanism for the emergence of an additional band gap due to a Ti-O-C bond in the TiO2-graphene hybrid system for enhanced photodegradation of methylene blue under visible light. RSC Adv. 2014, 4, 59890–59901. [Google Scholar] [CrossRef]
- Chun, W.J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, H. Theoretical Investigation of Ta2O5, TaON, and Ta3N5: Electronic Band Structures and Absolute Band Edges. J. Phys. Chem. C 2017, 121, 3241–3251. [Google Scholar] [CrossRef]
- Khanal, V.; Irani, R.; Fiechter, S.; Abdi, F.F.; Subramanian, V.R. The Photoelectrochemical and Photocatalytic Properties of Tantalum Oxide and Tantalum Nitride. J. Electrochem. Soc. 2019, 166, 1–7. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor−Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Phulé, P.P. Sol-gel synthesis of ferroelectric lithium tantalate ceramics: FTIR investigation of the molecular modification of tantalum ethoxide. J. Mater. Res. 1993, 8, 334–338. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, J.; Jiao, S.; Huang, K.; Zhu, H. In situ chemical reduction of the Ta3N5 quantum dots coupled TaON hollow spheres heterojunction photocatalyst for water oxidation. J. Mater. Chem. 2012, 22, 21972. [Google Scholar] [CrossRef]
- Stampfl, C.; Freeman, A.J. Stable and metastable structures of the multiphase tantalum nitride system. Phys. Rev. B 2005, 71, 024111. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Domen, K. Electrochemical behavior of thin Ta3N5 semiconductor film. J. Phys. Chem. B 2004, 108, 11049–11053. [Google Scholar] [CrossRef]
- Brauer, G.; Weidlein, J.R. Synthesis and Properties of Tantalum Oxide Nitride, TaON. Angew. Chem. Int. Ed. 1965, 4, 875. [Google Scholar] [CrossRef]
- Orhan, E.; Tessier, F.; Marchand, R. Synthesis and energetics of yellow TaON. Solid State Sci. 2002, 4, 1071–1076. [Google Scholar] [CrossRef]
- De Respinis, M.; Fravventura, M.; Abdi, F.F.; Schreuders, H.; Savenije, T.J.; Smith, W.A.; Dam, B.; van de Krol, R. Oxynitrogenography: Controlled Synthesis of Single-Phase Tantalum Oxynitride Photoabsorbers. Chem. Mater. 2015, 27, 7091–7099. [Google Scholar] [CrossRef]
- Ojha, M.; Dhiman, A.K. Problem, Failure and Safety Analysis of Ammonia Plant: A Review. Int. Rev. Chem. Eng. 2010, 2, 631–646. [Google Scholar]
- Gao, Q.; Giordano, C.; Antonietti, M. Controlled synthesis of tantalum oxynitride and nitride nanoparticles. Small 2011, 7, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Tokuyasu, Z.; Ono, Y.; Iwasaki, M.; Ito, S. Synthetic conditions and color characteristics of tantalum oxynitride prepared via liquid-NH3 process. Adv. Mater. Sci. Eng. 2009, 2009, 2–6. [Google Scholar] [CrossRef] [Green Version]










| Catalyst | Adsorption Contribution (%) | Photocatalysis Contribution (%) |
|---|---|---|
| Ta2O5 | ~5 | ~60 |
| TaON | 20 | 60 |
| Ta3N5 | 40 | 58 |
| Sample | Efb, V |
|---|---|
| Ta2O5 (As-synthesized) | −0.63 |
| TaON | −0.95 |
| Ta3N5 | −0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, V.; Soto-Harrison, E.; Chandra, D.; Balayeva, N.O.; Bahnemann, D.W.; Subramanian, V. A Selective Synthesis of TaON Nanoparticles and Their Comparative Study of Photoelectrochemical Properties. Catalysts 2020, 10, 1128. https://doi.org/10.3390/catal10101128
Khanal V, Soto-Harrison E, Chandra D, Balayeva NO, Bahnemann DW, Subramanian V. A Selective Synthesis of TaON Nanoparticles and Their Comparative Study of Photoelectrochemical Properties. Catalysts. 2020; 10(10):1128. https://doi.org/10.3390/catal10101128
Chicago/Turabian StyleKhanal, Vijay, Eric Soto-Harrison, Dhanesh Chandra, Narmina O. Balayeva, Detlef W. Bahnemann, and Vaidyanathan (Ravi) Subramanian. 2020. "A Selective Synthesis of TaON Nanoparticles and Their Comparative Study of Photoelectrochemical Properties" Catalysts 10, no. 10: 1128. https://doi.org/10.3390/catal10101128
APA StyleKhanal, V., Soto-Harrison, E., Chandra, D., Balayeva, N. O., Bahnemann, D. W., & Subramanian, V. (2020). A Selective Synthesis of TaON Nanoparticles and Their Comparative Study of Photoelectrochemical Properties. Catalysts, 10(10), 1128. https://doi.org/10.3390/catal10101128