Enhanced Carbon Dioxide Decomposition Using Activated SrFeO3−δ
Abstract
:1. Introduction
2. Results
2.1. Characterization
2.2. Oxygen-Deficient SrFeO3−δ
2.3. Effect of Conductivity on CO2 Decomposition
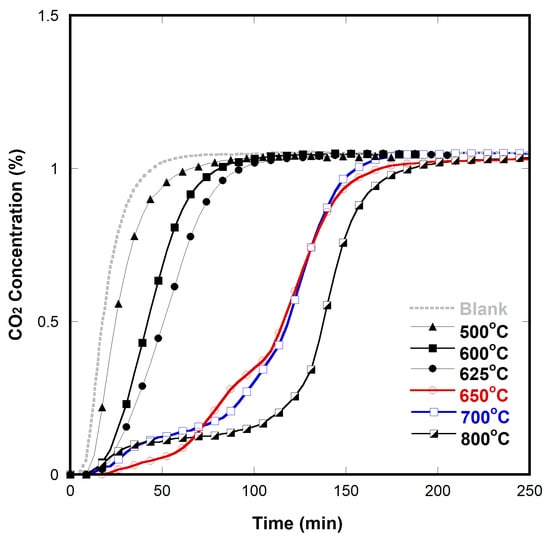
2.4. CO2 Decomposition
2.5. Stability Tests
3. Materials and Methods
3.1. SrFeO3−δ Preparation and Characterization
3.2. CO2 Decomposition Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nat. Cell Biol. 2009, 458, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.H. Potential sources of CO2 and the options for its large-scale utilization now and in the future. Catal. Today 1995, 23, 59–66. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Summary for policymakers: Global warming of 1.5 °C. IPCC 2018, 1, 1–32. [Google Scholar]
- Bergman, P.D.; Winter, E.M. Disposal of carbon dioxide in aquifers in the U.S. Energy Convers. Manag. 1995, 36, 523–526. [Google Scholar] [CrossRef]
- Rubin, E.S.; Rao, A.B. A Technical, Economic and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. In A Technical, Economic and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2002; Volume 36, pp. 4467–4475. [Google Scholar]
- Desideri, U.; Paolucci, A. Performance modelling of a carbon dioxide removal system for power plants. Energy Convers. Manag. 1999, 40, 1899–1915. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Mark, M.F.; Maier, W.F. CO2-Reforming of methane on supported Rh and Ir catalysts. J. Catal. 1996, 164, 122–130. [Google Scholar] [CrossRef]
- Tamaura, Y.; Tahata, M. Complete reduction of carbon dioxide to carbon using cation-excess magnetite. Nat. Cell Biol. 1990, 346, 255–256. [Google Scholar] [CrossRef]
- Shin, H.C.; Choi, S.C.; Jung, K.D.; Han, S.H. Mechanism of M ferrites (M = Cu and Ni) in the CO2 Decomposition reaction. Chem. Mater. 2001, 13, 1238–1242. [Google Scholar] [CrossRef]
- Tabata, M.; Nishida, Y.; Kodama, T.; Mimori, K.; Yoshida, T.; Tamaura, Y. CO2 decomposition with oxygen-deficient Mn(II) ferrite. J. Mater. Sci. 1993, 28, 971–974. [Google Scholar] [CrossRef]
- Kodama, T.; Tabata, M.; Tominaga, K.; Yoshida, T.; Tamaura, Y. Decomposition of CO2 and CO into carbon with active wustite prepared form Zn(II)-bearing ferrite. J. Mater. Sci. 1993, 28, 547–552. [Google Scholar] [CrossRef]
- Shin, H.C.; Oh, J.H.; Lee, J.C.; Han, S.H.; Choi, S.C. The carbon dioxide decomposition reaction with (NixCu1-x)Fe2O4 solid solution. Phys. Stat. Sol. A 2002, 189, 741–745. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.R. Characterization of wet processed (Ni, Zn)-ferrites for CO2 decomposition. J. Mater. Sci. 2001, 36, 4813–4816. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.R.; Lee, C.W.; Murakami, Y.; Shindo, D. Morphological properties of ultra-fine (Ni,Zn)-ferrites and their ability to decompose CO2. J. Mater. Chem. 2001, 11, 3373–3376. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, J.T.; Sim, J.; Lee, J.H.; Nam, S.C.; Park, C.Y. Carbon dioxide decomposition using SrFeCo0.5Ox, a nonperovskite-type metal oxide. J. CO2 Util. 2019, 34, 709–715. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.; Liu, X.; Shen, Y.; Xu, L. Bismuth doping effects on the structure, electrical conductivity and oxygen permeability of Ba0.6Sr0.4Co0.7Fe0.3O3−δ ceramic membranes. Int. J. Hydrogen Energy 2012, 37, 12694–12699. [Google Scholar] [CrossRef]
- Li, X.; Kerstiens, T.; Markus, T. Oxygen permeability and phase stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite at intermediate temperatures. J. Membr. Sci. 2013, 438, 83–89. [Google Scholar] [CrossRef]
- Kovalevsky, A. Processing and oxygen permeability of asymmetric ferrite-based ceramic membranes. Solid State Ionics 2008, 179, 61–65. [Google Scholar] [CrossRef]
- Leo, A.; Liu, S.; Da Costa, J.C.D. Development of mixed conducting membranes for clean coal energy delivery. Int. J. Greenh. Gas Control. 2009, 3, 357–367. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Chemin 2004, 35. [Google Scholar] [CrossRef]
- Fuks, D.; Mastrikov, Y.A.; Kotomin, E.A.; Maier, J. Ab initio thermodynamic study of (Ba,Sr)(Co,Fe)O3 perovskite solid solutions for fuel cell applications. J. Mater. Chem. A 2013, 1, 14320. [Google Scholar] [CrossRef]
- Falcón, H.; Barbero, J.A.; Alonso, J.A.; Martínez-Lope, M.J.; Fierro, J.L.G. SrFeO3−δ Perovskite oxides: Chemical features and performance for methane combustion. Chem. Mater. 2002, 14, 2325–2333. [Google Scholar] [CrossRef]
- Marek, E.; Hu, W.; Gaultois, M.; Grey, C.P.; Scott, S.A. The use of strontium ferrite in chemical looping systems. Appl. Energy 2018, 223, 369–382. [Google Scholar] [CrossRef]
- Takeda, Y.; Kanno, K.; Takada, T.; Yamamoto, O.; Takano, M.; Nakayama, N.; Bando, Y. Phase relation in the oxygen nonstoichiometric system, SrFeOx (2.5 ≤ × ≤ 3.0). J. Solid State Chem. 1986, 63, 237–249. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Wang, S.; Komvokis, V.G.; Amiridis, M.D.; Heyden, A.; Ma, S.; Chen, F. Synthesis and characterization of Mo-doped SrFeO3−δ as cathode materials for solid oxide fuel cells. J. Power Sources 2012, 202, 63–69. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Zhang, L.; Wang, F.; Jiang, H.; Au, C.T. Three-dimensionally ordered macroporous SrFeO3−δ with high surface area: Active catalysts for the complete oxidation of toluene. Appl. Catal. A Gen. 2012, 425, 153–160. [Google Scholar] [CrossRef]
- Hombo, J.; Matsumoto, Y.; Kawano, T. Electrical conductivities of SrFeO3−δ and BaFeO3−δ perovskites. J. Solid State Chem. 1990, 84, 138–143. [Google Scholar] [CrossRef]
- Hodges, J.; Short, S.; Jorgensen, J.; Xiong, X.; Dabrowski, B.; Mini, S.; Kimball, C. Evolution of oxygen-vacancy ordered crystal structures in the perovskite series SrnFenO3n−1 (n = 2, 4, 8, and ∞), and the relationship to electronic and magnetic properties. J. Solid State Chem. 2000, 151, 190–209. [Google Scholar] [CrossRef]
- Khare, A.; Lee, J.; Park, J.; Kim, G.Y.; Choi, S.Y.; Katase, T.; Roh, S.; Yoo, T.S.; Hwang, J.; Ohta, H.; et al. Directing oxygen vacancy channels in SrFeO2.5 epitaxial thin films. ACS Appl. Mater. Interfaces 2018, 10, 4831–4837. [Google Scholar] [CrossRef]
- Vashuk, V.V.; Kokhanovskii, L.V.; Yushkevich, I.I. Electrical conductivity and oxygen stoichiometry of SrFeO3−δ. Inorg. Mater. 2000, 36, 79–83. [Google Scholar] [CrossRef]
- Ma, B.; Victory, N.I.; Balachandran, U.; Mitchell, B.J.; Richardson, J.W. Study of the mixed-conducting SrFeCo0.5Oy system. J. Am. Ceram. Soc. 2004, 85, 2641–2645. [Google Scholar] [CrossRef]
- Park, C.; Lee, T.; Dorris, S.; Park, J.H.; Balachandran, U. Ethanol reforming using Ba0.5Sr0.5Cu0.2Fe0.8O3−δ/Ag composites as oxygen transport membranes. J. Power Sources 2012, 214, 337–343. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and Its Application; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1984. [Google Scholar]






| Sample | Decomposed CO2 (mmol/g) | Produced CO (mmol/g) | Reference |
|---|---|---|---|
| NiFe2O4−δ | 2.25 | 2.89 | [17] |
| SrFeCo0.5Ox | 2.35 | 2.65 | [17] |
| SrFeO3−δ | 3.30 | 2.95 | This work |
| Temperature (°C) | Cycle (Number) | Decomposed CO2 (mmol/g) | Produced CO (mmol/g) | Cell Parameters (Å) |
|---|---|---|---|---|
| 700 | 1 | 1.40 | 1.00 | a = 5.66(8) b = 15.59(2) c = 5.53(4) V = 489.1 Å3 |
| 2 | 1.40 | 1.03 | ||
| 3 | 1.39 | 1.03 | ||
| 4 | 1.42 | 1.04 | ||
| 5 | 1.29 | 0.98 | ||
| Average | 1.38 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Kim, S.-H.; Kim, J.-Y.; Lee, K.B.; Nam, S.-C.; Park, C.Y. Enhanced Carbon Dioxide Decomposition Using Activated SrFeO3−δ. Catalysts 2020, 10, 1278. https://doi.org/10.3390/catal10111278
Sim J, Kim S-H, Kim J-Y, Lee KB, Nam S-C, Park CY. Enhanced Carbon Dioxide Decomposition Using Activated SrFeO3−δ. Catalysts. 2020; 10(11):1278. https://doi.org/10.3390/catal10111278
Chicago/Turabian StyleSim, Jaeyong, Sang-Hyeok Kim, Jin-Yong Kim, Ki Bong Lee, Sung-Chan Nam, and Chan Young Park. 2020. "Enhanced Carbon Dioxide Decomposition Using Activated SrFeO3−δ" Catalysts 10, no. 11: 1278. https://doi.org/10.3390/catal10111278
APA StyleSim, J., Kim, S. -H., Kim, J. -Y., Lee, K. B., Nam, S. -C., & Park, C. Y. (2020). Enhanced Carbon Dioxide Decomposition Using Activated SrFeO3−δ. Catalysts, 10(11), 1278. https://doi.org/10.3390/catal10111278