Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR
Abstract
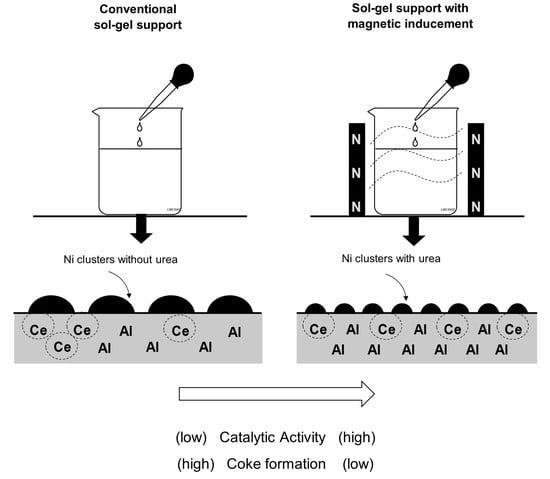
:1. Introduction
2. Results
2.1. Effect of CeO2–Al2O3 Support Prepared with and without Magnetic Inducement
2.1.1. Effect on H2, CO2 and CO Production
2.1.2. Effect on Coke Reduction
2.1.3. Stability Test
2.2. Catalyst and Support Characterization
3. Conclusions
4. Materials and Methods
4.1. Support Preparation
4.2. Catalyst Preparation
4.3. Ethanol Steam Reforming Process
4.4. Support Characterization
4.5. Catalyst Characterization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benito, M.; Sanz, J.; Isabel, R.; Padilla, R.; Arjona, R.; Daza, L. Bio-ethanol steam reforming: Insights on the mechanism for hydrogen production. J. Power Sources 2005, 151, 11–17. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Arslan, A.; Doğu, T. Effect of calcination/reduction temperature of Ni impregnated CeO2–ZrO2 catalysts on hydrogen yield and coke minimization in low temperature reforming of ethanol. Int. J. Hydrog. Energy 2016, 41, 16752–16761. [Google Scholar] [CrossRef]
- Kumbilieva, K.; Petrov, L.; Alhamed, Y.A.; Alzahrani, A. Reaction Mechanism and Deactivation Modes of Heterogeneous Catalytic Systems. Chin. J. Catal. 2011, 32, 387–404. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction pathway for ethanol steam reforming on a Ni/SiO2 catalyst including coke formation. Int. J. Hydrog. Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.K. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Kugai, J.; Velu, S.; Song, C. Low-temperature reforming of ethanol over CeO2-supported Ni-Rh bimetallic catalysts for hydrogen production. Catal. Lett. 2005, 101, 255–264. [Google Scholar] [CrossRef]
- Cavallaro, S. Low-temperature reforming of ethanol over CeO2-supported Ni-Rh bimetallic catalysts for hydrogen production. Energy Fuels 2000, 14, 1195–1199. [Google Scholar] [CrossRef]
- Auprêtre, F.; Descorme, C.; Duprez, D. Bio-ethanol catalytic steam reforming over supported metal catalysts. Catal. Commun. 2002, 3, 263–267. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Valle, B.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Origin and Nature of Coke in Ethanol Steam Reforming and Its Role in Deactivation of Ni/La2O3−αAl2O3 Catalyst. Ind. Eng. Chem. Res. 2019, 58, 14736–14751. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-promoted Ni@Al2O3 core-shell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance. Int. J. Hydrog. 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Ang, M.; Mo, L.; Li, Z.; Oemar, U.; Kawi, S.-. Highly active and coke resistant Ni/SiO2 catalysts for oxidative reforming of model biogas: Effect of low ceria loading. J. CO2 Util. 2017, 19, 284–295. [Google Scholar] [CrossRef]
- Fard, A.A.; Arvaneh, R.; Alavi, S.M.; Bazyari, A.; Valaei, A. Propane steam reforming over promoted Ni–Ce/Mg Al2O4 catalysts: Effects of Ce promoter on the catalyst performance using developed CCD model. Int. J. Hydrog. 2019, 44, 21607–21622. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Ebiad, M.A.; El-Hafiz, D.R.A.; Elsalamony, R.A.; Mohamed, L.S. Ni supported high surface area CeO2–ZrO2 catalysts for hydrogen production from ethanol steam reforming. RSC Adv. 2012, 2, 8145–8156. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. ChemPhysChem 2003, 4, 1041–1047. [Google Scholar] [CrossRef]
- Fujiwara, M.; Chie, K.; Sawai, J.; Shimizu, D.; Tanimoto, Y. On the Movement of Paramagnetic Ions in an Inhomogeneous Magnetic Field. J. Phys. Chem. B 2004, 108, 3531–3534. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Santos, J.; Li, P.; Yu, J.-G.; Cunha, A.F.; Rodrigues, A.E. Simplified kinetic model for steam reforming of ethanol on a Ni/Al2O3 catalyst. Can. J. Chem. Eng. 2014, 92, 116–130. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Crooks, A.B.; Zhou, J. Effect of Ceria Support on the Structure of Ni Nanoparticles. J. Phys. Chem. Lett. 2010, 1, 1447–1453. [Google Scholar] [CrossRef]
- Chang, K.-T.; Weng, C.-I. An investigation into the structure of aqueous NaCl electrolyte solutions under magnetic fields. Comput. Mater. Sci. 2008, 43, 1048–1055. [Google Scholar] [CrossRef]








| Catalysts | Gaseous Production Rate (mmol/min·gcat) | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | 550 °C | 650 °C | ||||||
| H2 | CO2 | CO | CO2:CO | H2 | CO2 | CO | CO2:CO | |
| Ni/γ–Al2O3 | 672.1 ± 1.6 | 235.7 | 37.9 | 6.21 | 683.4 ± 5.7 | 231.0 | 71.0 | 3.27 |
| Ni/CeO2–Al2O3 | 680.1 ± 2.1 | 258.3 | 41.2 | 6.27 | 761.9 ± 2.8 | 276.7 | 38.8 | 7.13 |
| Ni/CeO2–Al2O3 (N–S) | 675.1 ± 3.6 | 255.0 | 39.1 | 6.52 | 727.7 ± 4.3 | 238.8 | 32.8 | 6.94 |
| Ni/CeO2–Al2O3 (N–N) | 773.2 ± 4.2 | 293.9 | 41.6 | 7.07 | 885.8 ± 5.4 | 336.3 | 24.9 | 9.13 |
| Ni/CeO2–Al2O3 (S–S) | 781.5 ± 3.3 | 299.5 | 37.2 | 8.06 | 813.6 ± 4.8 | 299.3 | 28.3 | 8.04 |
| Catalysts | Coke (mg/gcat) | ||
|---|---|---|---|
| 550 °C | 650 °C | 750 °C | |
| Ni/γ–Al2O3 | 1.46 | 2.41 | 4.97 |
| Ni/CeO2–Al2O3 | 1.32 | 2.13 | 4.08 |
| Ni/CeO2–Al2O3 (N–S) | 1.42 | 2.28 | 4.84 |
| Ni/CeO2–Al2O3 (N–N) | 1.25 | 1.71 | 2.82 |
| Ni/CeO2–Al2O3 (S–S) | 1.30 | 1.75 | 2.79 |
| Catalysts | Ni Content (wt %) | Ni Dispersion (%) | Ni Surface Area (m2/gNi) |
|---|---|---|---|
| Ni/γ–Al2O3 | 9.70 ± 0.15 | 1.0 | 7.3 |
| Ni/CeO2–Al2O3 | 9.60 ± 0.13 | 2.8 | 18.3 |
| Ni/CeO2–Al2O3 (N–S) | 9.82 ± 0.19 | 2.9 | 18.8 |
| Ni/CeO2–Al2O3 (N–N) | 9.86 ± 0.20 | 3.2 | 21.4 |
| Ni/CeO2–Al2O3 (S–S) | 9.62 ± 0.19 | 3.2 | 21.0 |
| Supports | Preparation Method | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (Å) | Lattice Constant of Al2O3 Framework (Å) | |
|---|---|---|---|---|---|---|
| Process | Magnetic Inducement | |||||
| γ–Al2O3 | Sol–gel | none | 165.3 | 0.39 | 95.1 | 7.9068 |
| CeO2–Al2O3 | none | 138.1 | 0.33 | 94.3 | 7.9141 | |
| N–S | 142.2 | 0.32 | 91.2 | 7.9145 | ||
| N–N | 135.6 | 0.33 | 96.3 | 7.9220 | ||
| S–S | 135.6 | 0.32 | 94.2 | 7.9180 | ||
| Supports | Magnetic Inducement | Ce:Al Molar Ratio × 102 | ||
|---|---|---|---|---|
| Range | Average | SD | ||
| CeO2–Al2O3 | none | 4.32–26.17 | 9.65 | 9.28 |
| N–S | 2.00–2.66 | 2.38 | 0.29 | |
| N–N | 7.20–7.98 | 7.58 | 0.33 | |
| S–S | 6.24–7.09 | 6.70 | 0.35 | |
| Support | Peak Energy (eV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al–O | Al2O3 | Ce 3d (3+) 5/2 | Ce 3d (3+) 3/2 | Ce 3d (4+) 5/2 | Ce 3d (4+) 3/2 | Ce 3d (sat) 5/2 | Ce 3d (sat) 3/2 | |||
| γ–Al2O3 | 73.64 | 75.08 | - | - | - | - | - | - | - | - |
| CeO2–Al2O3 | 73.84 | 75.06 | 882.12 | 900.22 | 884.53 | 897.79 | 902.63 | 916.23 | 888.36 | 908.31 |
| CeO2–Al2O3 (N–S) | 74.15 | 75.77 | 882.44 | 900.54 | 884.85 | 898.26 | 902.95 | 916.36 | 888.73 | 906.83 |
| CeO2–Al2O3 (N–N) | 73.86 | 75.23 | 882.04 | 900.14 | 884.33 | 897.73 | 902.43 | 916.31 | 888.07 | 907.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vacharapong, P.; Arayawate, S.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L.; Charojrochkul, S. Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR. Catalysts 2020, 10, 1357. https://doi.org/10.3390/catal10111357
Vacharapong P, Arayawate S, Katanyutanon S, Toochinda P, Lawtrakul L, Charojrochkul S. Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR. Catalysts. 2020; 10(11):1357. https://doi.org/10.3390/catal10111357
Chicago/Turabian StyleVacharapong, Pumiwat, Sirintra Arayawate, Sasimas Katanyutanon, Pisanu Toochinda, Luckhana Lawtrakul, and Sumittra Charojrochkul. 2020. "Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR" Catalysts 10, no. 11: 1357. https://doi.org/10.3390/catal10111357
APA StyleVacharapong, P., Arayawate, S., Katanyutanon, S., Toochinda, P., Lawtrakul, L., & Charojrochkul, S. (2020). Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR. Catalysts, 10(11), 1357. https://doi.org/10.3390/catal10111357