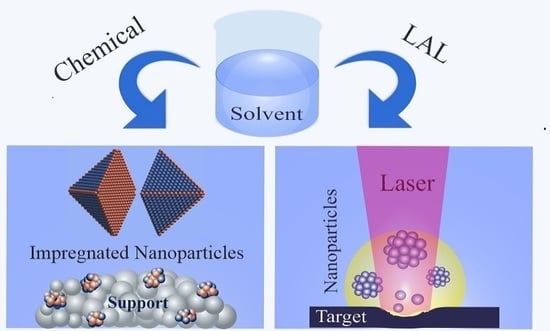
Chemical and Laser Ablation Synthesis of Monometallic and Bimetallic Ni-Based Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Wet-Chemical Synthesis of (Partially Oxidized) Ni and NiCu Nanoparticles on ZrO2
2.2. Nanosecond Laser Synthesis of Ni/NiOx Nanoparticles
2.3. Femtosecond Laser-Synthesis of Bimetallic (Oxidized) NiAu Nanoparticles
3. Materials and Methods
3.1. Wet-Chemical Synthesis of ZrO2 Supported (Oxidized) Ni and NiCu Nanoparticles
3.2. Nanosecond Pulsed Laser Ablation of Ni Target in Liquids
3.3. Femtosecond Pulsed Laser Ablation of NiAu Target in Liquids
3.4. Characterization Techniques (BF-, DF-TEM, SAED, HRTEM, EDX, XPS, IR)
3.4.1. Impregnated Ni and NiCu Nanoparticles
3.4.2. Laser-Synthesized (Oxidized) Ni and NiAu Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Sachtler, W. Selectivity and rate of activity decline of bimetallic catalysts. J. Mol. Catal. 1984, 25, 1–12. [Google Scholar] [CrossRef]
- Schwank, J. Bimetallic Catalysts: Discoveries, Concepts, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1983. [Google Scholar] [CrossRef] [Green Version]
- Toshima, N.; Harada, M.; Yamazaki, Y.; Asakura, K. Catalytic activity and structural analysis of polymer-protected gold-palladium bimetallic clusters prepared by the simultaneous reduction of hydrogen tetrachloroaurate and palladium dichloride. J. Phys. Chem. 1992, 96, 9927–9933. [Google Scholar] [CrossRef]
- Toshima, N.; Wang, Y. Novel preparation, characterization and catalytic properties of polymer-protected Cu/Pd bimetallic colloid. Chem. Lett. 1993, 22, 1611–1614. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Klötzer, B.; Mayr, L.; Rameshan, R.; Zemlyanov, D.; Bernardi, J.; Föttinger, K.; Rupprechter, G. Surface modification processes during methane decomposition on Cu-promoted Ni–ZrO2 catalysts. Catal. Sci. Technol. 2015, 5, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-temperature laser synthesis in liquid of oxide, metal-oxide core-shells, and doped oxide nanoparticles. Chemistry 2020, 26, 9206–9242. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Yeom, G.Y.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Lasemi, N.; Rentenberger, C.; Pospichal, R.; Cherevan, A.S.; Pfaffeneder-Kmen, M.; Liedl, G.; Eder, D. Femtosecond laser-assisted synthesis of Ni/Au BONs in various alcoholic solvents. Appl. Phys. A 2019, 125, 544. [Google Scholar] [CrossRef]
- Garfinkel, D.A.; Pakeltis, G.; Tang, N.; Ivanov, I.N.; Fowlkes, J.D.; Gilbert, D.A.; Rack, P.D. Optical and magnetic properties of Ag–Ni bimetallic nanoparticles assembled via pulsed laser-induced dewetting. ACS Omega 2020, 5, 19285–19292. [Google Scholar] [CrossRef] [PubMed]
- Bharati, M.S.S.; Byram, C.; Soma, V.R. Femtosecond laser fabricated Ag@Au and Cu@Au alloy nanoparticles for surface enhanced Raman spectroscopy based trace explosives detection. Front. Phys. 2018, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Nastulyavichus, A.A.; Kudryashov, I.S.; Smirnov, A.N.; Rudenko, A.A.; Kharin, A.Y.; Busleev, I.N.; Zayarny, A.D.; Ionin, A.A.; Kirilenko, A.D.; Brunkov, P.N. Novel approach of controllable stoichiometric fabrication of alloyed Au/Ag nanoparticles by nanosecond laser ablation of thin bi-layered films in water. Laser Phys. Lett. 2019, 16, 096002. [Google Scholar] [CrossRef]
- Kuladeep, R.; Jyothi, L.; Alee, K.S.; Deepak, K.L.N.; Rao, D.N. Laser-assisted synthesis of Au-Ag alloy nanoparticles with tunable surface plasmon resonance frequency. Opt. Mater. Express 2012, 2, 161–172. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Jakobi, J.; Winkel, A.; Stiesch, M.; Barcikowski, S. Alloying colloidal silver nanoparticles with gold disproportionally controls antibacterial and toxic effects. Gold Bull. 2013, 47, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Neumeister, A.; Jakobi, J.; Rehbock, C.; Moysig, J.; Barcikowski, S. Monophasic ligand-free alloy nanoparticle synthesis determinants during pulsed laser ablation of bulk alloy and consolidated microparticles in water. Phys. Chem. Chem. Phys. 2014, 16, 23671–23678. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of alloy nanoparticles by laser ablation of Au/Fe multilayer films in liquid environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Chen, C.Y.; Yang, C.C. Facile synthesis of bimetallic nanoparticles by femtosecond laser irradiation method. Arab. J. Chem. 2017, 10, S1395–S1401. [Google Scholar] [CrossRef] [Green Version]
- Moniri, S.; Hantehzadeh, M.; Ghoranneviss, M.; Asadabad, M.A. Au-Pt alloy nanoparticles obtained by nanosecond laser irradiation of gold and platinum bulk targets in an ethylene glycol solution. Eur. Phys. J. Plus 2017, 132, 318. [Google Scholar] [CrossRef]
- Tarasenko, N.V.; Butsen, A.V.; Nevar, E.A.; Rozantsev, V.A. Plasma assisted synthesis of bimetallic nanoparticles with laser-aided modification of their structure. Phys. Chem. Appl. Nanostruct. 2005, 501–504. [Google Scholar] [CrossRef]
- Machado, T.R.; Macedo, N.G.; Assis, M.; Doñate-Buendia, C.; Mínguez-Vega, G.; Teixeira, M.M.; Foggi, C.C.; Vergani, C.E.; Beltrán-Mir, H.; Andrés, J.; et al. From complex inorganic oxides to Ag–Bi nanoalloy: Synthesis by femtosecond laser irradiation. ACS Omega 2018, 3, 9880–9887. [Google Scholar] [CrossRef] [PubMed]
- Lasemi, N.; Bomatí Miguel, O.; Lahoz, R.; Lennikov, V.V.; Pacher, U.; Rentenberger, C.; Kautek, W. Laser-assisted synthesis of colloidal FeWxOy and Fe/FexOy nanoparticles in water and ethanol. ChemPhysChem 2018, 19, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniz-Miranda, M.; Gellini, C.; Giorgetti, E.; Margheri, G. Bifunctional Fe3O4/Ag nanoparticles obtained by two-step laser ablation in pure water. J. Colloid Interface Sci 2017, 489, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echegoyen, Y.; Suelves, I.; Lázaro, M.; Moliner, R.; Palacios, J. Hydrogen production by thermocatalytic decomposition of methane over Ni-Al and Ni-Cu-Al catalysts: Effect of calcination temperature. J. Power Sources 2007, 169, 150–157. [Google Scholar] [CrossRef]
- Ashok, J.; Subrahmanyam, M.; Venugopal, A. Hydrotalcite structure derived Ni–Cu–Al catalysts for the production of H2 by CH4 decomposition. Int. J. Hydrogen Energy 2008, 33, 2704–2713. [Google Scholar] [CrossRef]
- Cangiano, M.D.L.A.; Ojeda, M.; Carreras, A.C.; Gonzalez, J.A.; Ruiz, M.D.C. A study of the composition and microstructure of nanodispersed Cu–Ni alloys obtained by different routes from copper and nickel oxides. Mater. Charact 2010, 61, 1135–1146. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Kovács, G.; Kozlov, S.M.; Föttinger, K.; Bernardi, J.; Klötzer, B.; Neyman, K.M.; Rupprechter, G. Surface composition changes of CuNi-ZrO2 during methane decomposition: An operando NAP-XPS and density functional study. Catal. Today 2017, 283, 134–143. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chang, L.; Qin, Y. The doping effect of copper on the catalytic growth of carbon fibers from methane over a Ni/Al2O3 catalyst prepared from Feitknecht compound precursor. J. Catal 1998, 178, 76–83. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Mondragón Galicia, G.; Mendoza Anaya, D.; Palacios, J.; Angeles-Chavez, C.; Arenas-Alatorre, J. Synthesis and characterization of bimetallic Cu–Ni/ZrO2 Nanocatalysts: H2 production by oxidative steam reforming of methanol. Int. J. Hydrog. Energy 2008, 33, 4569–4576. [Google Scholar] [CrossRef]
- Arán-Ais, R.M.; Rizo, R.; Grosse, P.; Algara-Siller, G.; Dembélé, K.; Plodinec, M.; Lunkenbein, T.; Chee, S.W.; Cuenya, B.R. Imaging electrochemically synthesized Cu2O cubes and their morphological evolution under conditions relevant to CO2 electroreduction. Nat. Commun. 2020, 11, 3489. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Xu, C. Directed synthesis of well dispersed and highly active AuCu and AuNi nanoparticle catalysts. Catal. Sci. Technol 2016, 6, 7137–7150. [Google Scholar] [CrossRef]
- Vysakh, A.B.; Babu, C.L.; Vinod, C.P. Demonstration of synergistic catalysis in Au@Ni bimetallic core–Shell nanostructures. J. Phys. Chem. C 2015, 119, 8138–8146. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Oko, D.N.; Garbarino, S.; Imbeault, R.; Chaker, M.; Tavares, A.C.; Guay, D.; Ma, D. Preparation of PtAu alloy colloids by laser ablation in solution and their characterization. J. Phys. Chem. C 2012, 116, 13413–13420. [Google Scholar] [CrossRef]
- Garcia, C.; Truttmann, V.; Lopez, I.; Haunold, T.; Marini, C.; Rameshan, C.; Pittenauer, E.; Kregsamer, P.; Dobrezberger, K.; Stöger-Pollach, M.; et al. Dynamics of Pd dopant atoms inside Au nanoclusters during catalytic CO oxidation. J. Phys. Chem. C 2020, 124, 23626–23636. [Google Scholar] [CrossRef]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a surface alloy catalyst for steam reforming. Science 1998, 279, 1913–1915. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Neofytidis, C.S.; Neophytides, S. Effect of Au and/or Mo doping on the development of carbon and sulfur tolerant anodes for SOFCs—A short review. Front. Environ. Sci 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.H.C.; King, D.L.; Roh, H.-S.; Wang, Y.; Heald, S.M. Structure and reactivity investigations on supported bimetallic AuNi catalysts used for hydrocarbon steam reforming. J. Catal. 2006, 244, 153–162. [Google Scholar] [CrossRef]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454. [Google Scholar] [CrossRef]
- Lam, J.; Amans, D.; Chaput, F.; Diouf, M.; LeDoux, G.; Mary, N.; Masenelli-Varlot, K.; Motto-Ros, V.; Dujardin, C. γ-Al2O3 nanoparticles synthesised by pulsed laser ablation in liquids: A plasma analysis. Phys. Chem. Chem. Phys. 2014, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.Y.; Wu, C.; Shugaev, M.V.; He, M. Atomistic modeling of nanoparticle generation in short pulse laser ablation of thin metal films in water. J. Colloid Interface Sci. 2017, 489, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Maiman, T.H. Stimulated optical radiation in ruby. Nat. Cell Biol. 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Patil, P.P.; Phase, D.M.; Kulkarni, S.K.; Ghaisas, S.V.; Kanetkar, S.M.; Ogale, S.B.; Bhide, V.G. Pulsed-laser–induced reactive quenching at liquid-solid interface: Aqueous oxidation of iron. Phys. Rev. Lett. 1987, 58, 238–241. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation and Size Control of Silver Nanoparticles by Laser Ablation in Aqueous Solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 2003, 94, 7941. [Google Scholar] [CrossRef] [Green Version]
- Brikas, M.; Barcikowski, S.; Chichkov, B.; Račiukaitis, G. Production of nanoparticles with high repetition rate picosecond laser JLMN. J. Laser Micro/Nanoeng. 2007, 2, 230–233. [Google Scholar] [CrossRef]
- Zimmer, K. Laser Processing and Chemistry. Z. Phys. Chem. 1999, 208, 291–292. [Google Scholar] [CrossRef]
- Singh, S.C.; Zeng, H.B.; Guo, C.; Cai, W. Nanomaterials: Processing and Characterization with Lasers; Wiley-VCH publication: Weinheim, Germany, 2012. [Google Scholar]
- Yang, G. Laser Ablation in Liquids, Principles and Applications in the Preparation of Nanomaterials; Pan Stanford Publishing: Stanford, CA, USA, 2012. [Google Scholar]
- Lasemi, N.; Pacher, U.; Rentenberger, C.; Bomatí-Miguel, O.; Kautek, W. Laser-assisted synthesis of colloidal Ni/NiOx core/shell nanoparticles in water and alcoholic solvents. ChemPhysChem 2017, 18, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Lasemi, N.; Pacher, U.; Zhigilei, L.; Bomatí-Miguel, O.; Lahoz, R.; Kautek, W. Pulsed laser ablation and incubation of nickel, iron and tungsten in liquids and air. Appl. Surf. Sci 2018, 433, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Rupprechter, G. Sum Frequency Generation and Polarization–Modulation Infrared Reflection Absorption Spectroscopy of Functioning Model Catalysts from Ultrahigh Vacuum to Ambient Pressure; Elsevier BV: Amsterdam, The Netherlands, 2007; Volume 51, pp. 133–263. [Google Scholar]
- Koper, M.T. Structure sensitivity and nanoscale effects in electrocatalysis. Nanoscale 2011, 3, 2054–2073. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Spiel, C.; Schmid, M.; Stöger-Pollach, M.; Schlögl, R.; Suchorski, Y.; Rupprechter, G. The role of defects in the local reaction kinetics of CO oxidation on low-index Pd surfaces. J. Phys. Chem. C 2013, 117, 12054–12060. [Google Scholar] [CrossRef]
- Datler, M.; Bespalov, I.; Rupprechter, G.; Suchorski, Y. Analysing the reaction kinetics for individual catalytically active components: CO oxidation on a Pd powder supported by Pt foil. Catal. Lett. 2015, 145, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Central Sci. 2016, 2, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Suchorski, Y.; Rupprechter, G. Local reaction kinetics by imaging. Surf. Sci. 2016, 643, 52–58. [Google Scholar] [CrossRef] [Green Version]
- King, M.E.; Personick, M.L. Defects by design: Synthesis of palladium nanoparticles with extended twin defects and corrugated surfaces. Nanoscale 2017, 9, 17914–17921. [Google Scholar] [CrossRef]
- Cheng, T.; Huang, Y.; Xiao, H.; Goddard, W.A. Predicted structures of the active sites responsible for the improved reduction of carbon dioxide by gold nanoparticles. J. Phys. Chem. Lett. 2017, 8, 3317–3320. [Google Scholar] [CrossRef] [Green Version]
- Suchorski, Y.; Kozlov, S.M.; Bespalov, I.; Datler, M.; Vogel, D.; Budinska, Z.; Neyman, K.M.; Rupprechter, G. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation. Nat. Mater. 2018, 17, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Bornovski, R.; Huang, L.-F.; Komarala, E.P.; Rondinelli, J.M.; Rosen, B.A. Catalytic enhancement of CO oxidation on LaFeO3 regulated by ruddlesden–popper stacking faults. ACS Appl. Mater. Interfaces 2019, 11, 33850–33858. [Google Scholar] [CrossRef] [PubMed]
- Elechiguerra, J.L.; Reyes-Gasga, J.; José-Yacamán, M. The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 2006, 16, 3906–3919. [Google Scholar] [CrossRef]
- Gammer, C.; Mangler, C.; Rentenberger, C.; Karnthaler, H. Quantitative local profile analysis of nanomaterials by electron diffraction. Scr. Mater. 2010, 63, 312–315. [Google Scholar] [CrossRef]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlögl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 Catalysts for Methanol Synthesis. Angew. Chem. Int. Ed. 2007, 46, 7324–7327. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.Y.; Feng, Y.; Dong, C.K.; Liu, H.; Du, X.W. A silver catalyst activated by stacking faults for the hydrogen evolution reaction. Nat. Catal. 2019, 2, 1107–1114. [Google Scholar] [CrossRef]
- Lasemi, N.; Rentenberger, C.; Liedl, G.; Eder, D. The influence of the fluid nature on femtosecond laser ablation properties of a SiO2/Si target and synthesis of ultrafine-grained Si nanoparticles. Nanoscale Adv. 2020, 2, 3991–4002. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Mazur, E. Inducing and probing non-thermal transitions in semiconductors using femtosecond laser pulses. Nat. Mater. 2002, 1, 217–224. [Google Scholar] [CrossRef]
- Amans, D.; Diouf, M.; Lam, J.; LeDoux, G.; Dujardin, C. Origin of the nano-carbon allotropes in pulsed laser ablation in liquids synthesis. J. Colloid Interface Sci. 2017, 489, 114–125. [Google Scholar] [CrossRef]
- Anic, K.; Wolfbeisser, A.; Li, H.; Rameshan, C.; Föttinger, K.; Bernardi, J.; Rupprechter, G. Surface spectroscopy on UHV-grown and technological Ni–ZrO2 reforming catalysts: From UHV to operando conditions. Top. Catal. 2016, 59, 1614–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprunger, P.T.; Besenbacher, F.; Stensgaard, I. STM investigation of the Ni(111)−c(4 × 2)−2CO structure. Chem. Phys. Lett. 1995, 243, 439–444. [Google Scholar] [CrossRef]
- Becker, L.; Aminpirooz, S.; Hillert, B.; Pedio, M.; Haase, J.; Adams, D.L. Threefold-coordinated hollow adsorption site for Ni(111)-c(4×2)-CO: A surface-extended x-ray-absorption fine-structure study. Phys. Rev. B 1993, 47, 9710–9714. [Google Scholar] [CrossRef] [PubMed]
- Dávila, M.E.; Asensio, M.; Woodruff, D.P.; Schindler, K.-M.; Hofmann, P.; Weiss, K.-U.; Dippel, R.; Gardner, P.; Fritzsche, V.; Bradshaw, A.; et al. Structure determination of Ni(111)c(4 × 2)-CO and its implications for the interpretation of vibrational spectroscopic data. Surf. Sci. 1994, 311, 337–348. [Google Scholar] [CrossRef]
- Kasai, P.H.; Bishop, R.J.; McLeod, D. Ligand effects on the redox reactions in nickel- and copper-exchanged zeolites. J. Phys. Chem. 1978, 82, 279–285. [Google Scholar] [CrossRef]
- Kitla, A.; Safonova, O.V.; Föttinger, K. Infrared studies on bimetallic copper/nickel catalysts supported on zirconia and ceria/zirconia. Catal. Lett. 2013, 143, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.M.; Aranda, D.A.G.; Schmal, M. Reforming of methane with carbon dioxide over Pt/ZrO2/Al2O3 Catalysts. J. Catal. 2001, 204, 498–511. [Google Scholar] [CrossRef]
- Daturi, M.; Binet, C.; LaValley, J.C.; Galtayries, A.; Sporken, R. Surface investigation on CexZr1-xO2 compounds. Phys. Chem. Chem. Phys. 1999, 1, 5717–5724. [Google Scholar] [CrossRef]
- Mihaylov, M.; Chakarova, K.; Hadjiivanov, K. Formation of carbonyl and nitrosyl complexes on titania- and zirconia-supported nickel: FTIR spectroscopy study. J. Catal. 2004, 228, 273–281. [Google Scholar] [CrossRef]
- Morterra, C.; Giamello, E.; Cerrato, G.; Centi, G.; Perathoner, S. Role of surface hydration state on the nature and reactivity of copper ions in Cu-ZrO2 catalysts: N2O decomposition. J. Catal. 1998, 179, 111–128. [Google Scholar] [CrossRef]
- Chen, S.; Zou, H.; Liu, Z.; Lin, W. DRIFTS study of different gas adsorption for CO selective oxidation on Cu-Zr-Ce-O Catalysts. Appl. Surf. Sci. 2009, 255, 6963–6967. [Google Scholar] [CrossRef]
- Zecchina, A. Infrared spectroscopy of adsorbed species on the surface of transition metal oxides. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 823–824. [Google Scholar] [CrossRef]
- Manzoli, M.; Di, M.R.; Boccuzzi, F.; Coluccia, S.; Kaspar, J. CO oxidation over CuOx-CeO2-ZrO2 catalysts: Transient behaviour and role of copper clusters in contact with ceria. Appl. Catal. B 2005, 61, 192–205. [Google Scholar] [CrossRef]
- Dalmon, J.A.; Primet, M.; Martin, G.A.; Imelik, B. Magnetic and infrared study of carbon monoxide chemisorption of silica supported nickel-copper alloys. Surf. Sci. 1975, 50, 95–108. [Google Scholar] [CrossRef]
- Guczi, L.; Stefler, G.; Geszti, O.; Sajó, I.; Pászti, Z.; Tompos, A.; Schay, Z. Methane dry reforming with CO2: A study on surface carbon species. Appl. Catal. A Gen. 2010, 375, 236–246. [Google Scholar] [CrossRef]
- Triantafyllopoulos, N.; Neophytides, S. Dissociative adsorption of CH4 on NiAu/YSZ: The nature of adsorbed carbonaceous species and the inhibition of graphitic C formation. J. Catal. 2006, 239, 187–199. [Google Scholar] [CrossRef]
- Chia-Ching, W.; Cheng-Fu, Y. Investigation of the properties of nanostructured Li-doped NiO films using the modified spray pyrolysis method. Nanoscale Res. Lett. 2013, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy C. Surf. Interface Anal. 1981, 3, 190–195. [Google Scholar] [CrossRef]
- Pawelec, B.; Damyanova, S.; Arishtirova, K.; Fierro, J.; Petrov, L. Structural and surface features of PtNi catalysts for reforming of methane with CO2. Appl. Catal. A Gen. 2007, 323, 188–201. [Google Scholar] [CrossRef]
- Jones, S.D.; Neal, L.M.; Hagelin-Weaver, H.E. Steam Reforming of Methanol Using Cu-ZnO Catalysts Supported on Nanoparticle Alumina. Appl. Catal. B 2008, 84, 631–642. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van, E.J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic Structure of Cuprous and Cupric Oxides. Phys. Rev. B Condens. Matter 1988, 38, 11322–11330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO supported on tetragonal ZrO2 catalysts for N2O decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; West, A.; Cheung, K.; Yu, K.-M.; Tsang, S.C.E. Dramatic effects of gallium promotion on methanol steam reforming Cu-ZnO catalyst for hydrogen production: Formation of 5 Å copper clusters from Cu-ZnGaOx. ACS Catal. 2013, 3, 1231–1244. [Google Scholar] [CrossRef]
- Chen, L.C.; Lin, S.D. The ethanol steam reforming over Cu-Ni/SiO2 catalysts: Effect of Cu/Ni ratio. Appl. Catal. B 2011, 106, 639–649. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, M.Y. Specific solvent produces specific phase Ni nanoparticles: A pulsed laser ablation in solvents. J. Phys. Chem. C 2014, 118, 14647–14654. [Google Scholar] [CrossRef]
- Safa, M.; Dorranian, D.; Masoudi, A.A.; Matin, L.F. Characterizing nickel oxide nanostructures produced by laser ablation method: Effects of laser fluence. Appl. Phys. A 2019, 125, 687. [Google Scholar] [CrossRef]
- Lasemi, N. Pulsed laser generation of colloidal nickel, iron, and tungsten-iron-oxide alloy core-shell nanoparticles. Ph.D. Thesis, Vienna University, Vienna, Austria, 2017. [Google Scholar]
- Ivanov, D.S.; Izgin, T.; Maiorov, A.N.; Veiko, V.P.; Rethfeld, B.; Dombrovska, Y.I.; Garcia, M.E.; Zavestovskaya, I.N.; Klimentov, S.M.; Kabashin, A.V. Numerical investigation of ultrashort laser-ablative synthesis of metal nanoparticles in liquids using the atomistic-continuum model. Molecules 2019, 25, 67. [Google Scholar] [CrossRef] [Green Version]
- Dorranian, D.; Eskandari, A.F. Effect of laser fluence on the characteristics of ZnO nanoparticles produced by laser ablation in acetone. Mol. Cryst. Liq. Cryst. 2015, 607, 1–12. [Google Scholar] [CrossRef]
- Fu, X.; Chen, B.; Tang, J.; Zewail, A.H. Photoinduced nanobubble-driven superfast diffusion of nanoparticles imaged by 4D electron microscopy. Sci. Adv. 2017, 3, e1701160. [Google Scholar] [CrossRef] [Green Version]
- Reich, S.; Schönfeld, P.; Wagener, P.; Letzel, A.; Ibrahimkutty, S.; Gökce, B.; Barcikowski, S.; Menzel, A.; Rolo, T.D.S.; Plech, A. Pulsed laser ablation in liquids: Impact of the bubble dynamics on particle formation. J. Colloid Interface Sci. 2017, 489, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.D.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci. Rep. 2015, 5, 16313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagener, P.; Ibrahimkutty, S.; Menzel, A.; Plech, A.; Barcikowski, S. Dynamics of silver nanoparticle formation and agglomeration inside the cavitation bubble after pulsed laser ablation in liquid. Phys. Chem. Chem. Phys. 2013, 15, 3068–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahimkutty, S.; Wagener, P.; Menzel, A.; Plech, A.; Barcikowski, S. Nanoparticle formation in a cavitation bubble after pulsed laser ablation in liquid studied with high time resolution small angle x-ray scattering. Appl. Phys. Lett. 2012, 101, 103104. [Google Scholar] [CrossRef] [Green Version]
- Tiemann, M.; Marlow, F.; Hartikainen, J.; Weiss, Ö.; Lindén, M. Ripening Effects in ZnS Nanoparticle Growth. J. Phys. Chem. C 2008, 112, 1463–1467. [Google Scholar] [CrossRef]
- Amendola, V.; Rizzi, G.A.; Polizzi, S.; Meneghetti, M. Synthesis of gold nanoparticles by laser ablation in toluene: Quenching and recovery of the surface plasmon absorption. J. Phys. Chem. B 2005, 109, 23125–23128. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Physics A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Bychkov, V.; Tyulenin, Y.; Firsova, A.A.; Shafranovsky, E.; Gorenberg, A.; Korchak, V.N. Carbonization of nickel catalysts and its effect on methane dry reforming. Appl. Catal. A Gen. 2013, 453, 71–79. [Google Scholar] [CrossRef]
- Olivié, G.; Giguère, D.; Vidal, F.; Ozaki, T.; Kieffer, J.C.; Nada, O.; Brunette, I. Wavelength dependence of femtosecond laser ablation threshold of corneal stroma. Opt. Express 2008, 16, 4121–4129. [Google Scholar] [CrossRef]
- Riabinina, D.; Chaker, M.; Margot, J. Dependence of gold nanoparticle production on pulse duration by laser ablation in liquid media. Nanotechnology 2012, 23, 135603. [Google Scholar] [CrossRef]
- Hu, A.; Sanderson, J.; Zaidi, A.; Wang, C.; Zhang, T.; Zhou, Y.; Duley, W. Direct synthesis of polyyne molecules in acetone by dissociation using femtosecond laser irradiation. Carbon 2008, 46, 1823–1825. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic nanoparticles of iron carbide, iron oxide, iron@iron oxide, and metal iron synthesized by laser ablation in organic solvents. J. Phys. Chem. C 2010, 115, 5140–5146. [Google Scholar] [CrossRef]
- Kwong, H.Y.; Wong, M.H.; Leung, C.W.; Wong, Y.W.; Wong, K.H. Formation of core/shell structured cobalt/carbon nanoparticles by pulsed laser ablation in toluene. J. Appl. Phys. 2010, 108, 34304. [Google Scholar] [CrossRef] [Green Version]
- Cristoforetti, G.; Pitzalis, E.; Spiniello, R.; Ishak, R.; Giammanco, F.; Muniz-Miranda, M.; Caporali, S. Physico-chemical properties of Pd nanoparticles produced by Pulsed Laser Ablation in different organic solvents. Appl. Surf. Sci. 2012, 258, 3289–3297. [Google Scholar] [CrossRef]
- Ilyin, A.; Golik, S.S. Femtosecond laser-induced breakdown spectroscopy of sea water. Spectrochim. Acta Part B: At. Spectrosc. 2013, 87, 192–197. [Google Scholar] [CrossRef]
- Fu, Q.; Bao, X. Confined microenvironment for catalysis control. Nat. Catal. 2019, 2, 834–836. [Google Scholar] [CrossRef]
- Prieto, M.J.; Klemm, H.W.; Xiong, F.; Gottlob, D.M.; Menzel, D.; Schmidt, T.; Freund, H.-J. Water formation under silica thin films: Real-time observation of a chemical reaction in a physically confined space. Angew. Chem. Int. Ed. 2018, 57, 8749–8753. [Google Scholar] [CrossRef] [Green Version]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Copéret, C.; Allouche, F.; Chang, K.W.; Conley, M.P.; Delley, M.F.; Fedorov, A.; Moroz, I.B.; Mougel, V.; Pucino, M.; Searles, K.; et al. Bridging the gap between industrial and well-defined supported catalysts. Angew. Chem. Int. Ed. 2018, 57, 6398–6440. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Mejía, C.H.; De Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Mantella, V.; Amoròs, L.C.; Buonsanti, R. Shaping non-noble metal nanocrystals via colloidal chemistry. Chem. Sci. 2020, 11, 11394–11403. [Google Scholar] [CrossRef]
- Rizo, R.; Cuenya, B.R. Shape-controlled nanoparticles as anodic catalysts in low-temperature fuel cells. ACS Energy Lett. 2019, 4, 1484–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Föttinger, K.; Schlögl, R.; Rupprechter, G. The mechanism of carbonate formation on Pd–Al2O3 catalysts. Chem. Commun. 2008, 3, 320–322. [Google Scholar] [CrossRef]
- Rupprechter, G.; Seeber, G.; Goller, H.; Hayek, K. Structure–activity correlations on Rh/Al2O3 and Rh/TiO2 thin film model catalysts after oxidation and reduction. J. Catal. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Penner, S.; Wang, D.; Se, D.S.; Rupprechter, G.; Podloucky, R.; Schlögl, R.; Hayek, K. Platinum nanocrystals supported by silica, alumina and ceria: Metal-support interaction due to high-temperature reduction in Hydrogen. Surf. Sci. 2003, 532–535, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Rupprechter, G. 8 Surface vibrational spectroscopy on noble metal catalysts from ultrahigh vacuum to atmospheric pressure. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2004, 100, 237–311. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, S.M.; Aleksandrov, H.A.; Goniakowski, J.; Neyman, K.M. Effect of MgO(100) support on structure and properties of Pd and Pt nanoparticles with 49-155 atoms. J. Chem. Phys. 2013, 139, 084701. [Google Scholar] [CrossRef]
- Rodriguez De La Fuente, O.; Borasio, M.; Galletto, P.; Rupprechter, G.; Freund, H.-J. The influence of surface defects on methanol decomposition on Pd(111) studied by XPS and PM-IRAS. Surf. Sci. 2004, 566, 740–745. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Morkel, M.; Unterhalt, H.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Rupprechter, G.; Freund, H.J. C–O bond scission on “defect-rich and perfect” Pd (1 1 1)? Surf. Sci. 2004, 566–568, 1024–1029. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Rupprechter, G. The flexible surface: Molecular studies explain the extraordinary diversity of surface chemical properties. J. Chem. Educ. 1998, 75, 161. [Google Scholar] [CrossRef]
- Yibin, X.; Masayoshi, Y.; Pierre, V. Inorganic materials database for exploring the nature of material. Jpn. J. Appl. Phys. 2011, 50, 11RH02. [Google Scholar]









| Medium | F (J cm−2) | Counted NPs | Mean (nm) | Median (nm) | IQR |
|---|---|---|---|---|---|
| Ethanol | 309 | 300 | 12.8 ± 8.7 | 10.6 | 1.0 |
| Ethanol | 486 | 381 | 17.1 ± 17.8 | 11.9 | 2.7 |
| Ethanol | 663 | 301 | 22.6 ± 20 | 16.9 | 4.4 |
| Ethanol | 840 | 268 | 27 ± 22.8 | 20.6 | 5.9 |
| Isopropanol | 663 | 482 | 16.6 ± 11.7 | 13.6 | 2.9 |
| Butanol | 663 | 477 | 14.5 ± 8.3 | 12.6 | 3.4 |
| Water | 663 | 175 | 17.7 ± 14.2 | 13.9 | 5.3 |
| Medium | Counted NPs | Mean (nm) | Median (nm) | IQR | Ni (a%) | Au (a%) | O (a%) | Ni/Au | Ni/O |
|---|---|---|---|---|---|---|---|---|---|
| Isopropanol | 965 | 14 ± 11.23 | 10.9 | 1.1 | 56.8 | 2.5 | 40.7 | 22.8 | 1.4 |
| Butanol | 811 | 17 ± 13.43 | 13.4 | 2.3 | 49.2 | 1.6 | 49.2 | 30.7 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasemi, N.; Rupprechter, G. Chemical and Laser Ablation Synthesis of Monometallic and Bimetallic Ni-Based Nanoparticles. Catalysts 2020, 10, 1453. https://doi.org/10.3390/catal10121453
Lasemi N, Rupprechter G. Chemical and Laser Ablation Synthesis of Monometallic and Bimetallic Ni-Based Nanoparticles. Catalysts. 2020; 10(12):1453. https://doi.org/10.3390/catal10121453
Chicago/Turabian StyleLasemi, Niusha, and Günther Rupprechter. 2020. "Chemical and Laser Ablation Synthesis of Monometallic and Bimetallic Ni-Based Nanoparticles" Catalysts 10, no. 12: 1453. https://doi.org/10.3390/catal10121453
APA StyleLasemi, N., & Rupprechter, G. (2020). Chemical and Laser Ablation Synthesis of Monometallic and Bimetallic Ni-Based Nanoparticles. Catalysts, 10(12), 1453. https://doi.org/10.3390/catal10121453