A Novel Route for Agarooligosaccharide Production with the Neoagarooligosaccharide-Producing β-Agarase as Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Feasibility of the Chemical-Biological Route
2.2. Preparation of A3, A5 by the Chemical-Biological Route
2.3. Purification of A3, A5
3. Materials and Methods
3.1. Materials
3.2. Acidolysis
3.3. Preparation of AgWH50B and DagA
3.4. Enzyme Assay
3.5. Preparation of A3, A5
3.6. Purification of A3, A5
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lahaye, M.; Yaphe, W.; Viet, M.T.P.; Rochas, C. 13 C-n.m.r. spectroscopic investigation of methylated and charged agarose oligosaccharides and polysaccharides. Carbohydr. Res. 1989, 190, 249–265. [Google Scholar] [CrossRef]
- Li, J.; Han, F.; Lu, X.; Fu, X.; Ma, C.; Chu, Y.; Yu, W. A simple method of preparing diverse neoagaro-oligosaccharides with β-agarase. Carbohydr. Res. 2007, 342, 1030–1033. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, L.; Yan, X.J. The preparation and bioactivity research of agaro-oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, W.; Hao, C.; Mao, X. Agaropentaose protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity through modulating NF-κB and p38MAPK signaling pathways. J. Funct. Foods 2019, 57, 222–232. [Google Scholar] [CrossRef]
- Ekborg, N.A.; Taylor, L.E.; Longmire, A.G.; Henrissat, B.; Weiner, R.M.; Hutcheson, S.W. Genomic and proteomic analyses of the agarolytic system expressed by Saccharophagus degradans 2–40. Appl. Environ. Microb. 2006, 72, 3396–3405. [Google Scholar] [CrossRef] [Green Version]
- Yun, E.J.; Kim, H.T.; Cho, K.M.; Yu, S.; Kim, S.; Choi, I.G.; Kim, K.H. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour. Technol. 2016, 199, 311–318. [Google Scholar] [CrossRef]
- Flament, D.; Barbeyron, T.; Jam, M.; Potin, P.; Czjzek, M.; Kloareg, B.; Michel, G. Alpha-agarases define a new family of glycoside hydrolases, distinct from beta-agarase families. Appl. Environ. Microb. 2007, 73, 4691–4694. [Google Scholar] [CrossRef] [Green Version]
- Potin, P. Purification and characterization of the alpha-agarase from Alteromonas agarlyticus (Cataldi) comb. nov. strain GJ1B. Eur. J. Biochem. 1993, 214, 599–607. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Jiang, C.; Mao, X. Biochemical characterization and substrate degradation mode of a novel alpha-agarase from Catenovulum agarivorans. J. Agric. Food Chem. 2019, 67, 10373–10379. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Seo, N.; Yu, S.; Kim, D.H.; Cho, K.M.; An, H.J.; Kim, J.H.; Choi, I.G.; Kim, K.H. Enzymatic liquefaction of agarose above the sol–gel transition temperature using a thermostable endo-type β-agarase, Aga16B. Appl. Microbiol. Biot. 2017, 101, 1111–1120. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, X.; Zhang, L.; Li, F.; Liu, Z.; Mao, X. Biochemical characterization and substrate degradation mode of a novel exotype beta-agarase from Agarivorans gilvus WH0801. J. Agric. Food Chem. 2017, 65, 7982–7988. [Google Scholar] [CrossRef]
- Bibb, M.J.; Jones, G.H.; Joseph, R.; Buttner, M.J.; Ward, J.M. The agarase gene (dag A) of Streptomyces coelicolor A3(2): Affinity purification and characterization of the cloned gene product. J. Gen. Appl. Microbiol. 1987, 133, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Rui, J.; Du, Z.; Xue, C.; Li, X.; Mao, X. Complete genome sequence of Agarivorans gilvus WH0801(T), an agarase-producing bacterium isolated from seaweed. J. Biotechnol. 2016, 219, 22–23. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef]
- Kazlowski, B.; Pan, C.L.; Ko, Y.T. Separation and quantification of neoagaro- and agaro-oligosaccharide products generated from agarose digestion by beta-agarase and HCl in liquid chromatography systems. Carbohydr. Res. 2008, 343, 2443–2450. [Google Scholar] [CrossRef]
- Kazlowski, B.; Pan, C.L.; Ko, Y.T. Monitoring and preparation of neoagaro- and agaro-oligosaccharide products by high performance anion exchange chromatography systems. Carbohydr. Polym. 2015, 122, 351–358. [Google Scholar] [CrossRef]
- Henry, R.W.; Pickard, D.W.; Hughes, P.E. Citric acid and fumaric acid as food additives for early-weaned piglets. Anim. Sci. 2010, 40, 505–509. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, L.; Lin, W.; Yan, X.J. Product monitoring and quantitation of oligosaccharides composition in agar hydrolysates by precolumn labeling HPLC. Talanta 2004, 64, 773–777. [Google Scholar] [CrossRef]
- Jang, M.-K.; Lee, D.-G.; Kim, N.; Yu, K.-H.; Jang, H.-J.; Lee, W.S.; Jang, J.H.; Lee, J.Y.; Lee, S.-H. Purification and characterization of neoagarotetraose from hydrolyzed agar. J. Microbiol. Biotechnol. 2009, 19, 1197–1200. [Google Scholar] [CrossRef]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Liu, N.; Yang, M.; Mao, X.; Mu, B.; Wei, D. Molecular cloning and expression of a new alpha-neoagarobiose hydrolase from Agarivorans gilvus WH0801 and enzymatic production of 3,6-anhydro-l-galactose. Biotechnol. Appl. Biochem. 2016, 63, 230–237. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, Z.; Huang, W.; Xue, C.; Mao, X. Coimmobilization of beta-agarase and alpha-neoagarobiose hydrolase for enhancing the production of 3,6-anhydro-l-galactose. J. Agric. Food Chem. 2018, 66, 7087–7095. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis.1. The mode of phage liberation by lysogenic Escherichia-coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef]

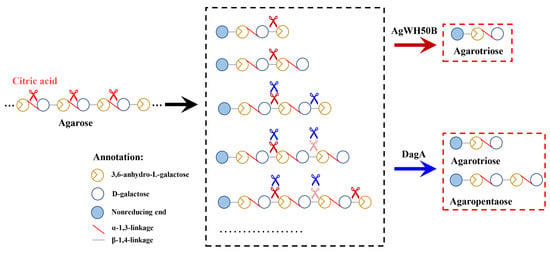
 ) and DagA (
) and DagA (  ) act on the A7 or A9 (b). The shades of scissors mean the priority of the hydrolyzed AOSs.
) act on the A7 or A9 (b). The shades of scissors mean the priority of the hydrolyzed AOSs.
 ) and DagA (
) and DagA (  ) act on the A7 or A9 (b). The shades of scissors mean the priority of the hydrolyzed AOSs.
) act on the A7 or A9 (b). The shades of scissors mean the priority of the hydrolyzed AOSs.





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. A Novel Route for Agarooligosaccharide Production with the Neoagarooligosaccharide-Producing β-Agarase as Catalyst. Catalysts 2020, 10, 214. https://doi.org/10.3390/catal10020214
Jiang C, Liu Z, Sun J, Xue C, Mao X. A Novel Route for Agarooligosaccharide Production with the Neoagarooligosaccharide-Producing β-Agarase as Catalyst. Catalysts. 2020; 10(2):214. https://doi.org/10.3390/catal10020214
Chicago/Turabian StyleJiang, Chengcheng, Zhen Liu, Jianan Sun, Changhu Xue, and Xiangzhao Mao. 2020. "A Novel Route for Agarooligosaccharide Production with the Neoagarooligosaccharide-Producing β-Agarase as Catalyst" Catalysts 10, no. 2: 214. https://doi.org/10.3390/catal10020214
APA StyleJiang, C., Liu, Z., Sun, J., Xue, C., & Mao, X. (2020). A Novel Route for Agarooligosaccharide Production with the Neoagarooligosaccharide-Producing β-Agarase as Catalyst. Catalysts, 10(2), 214. https://doi.org/10.3390/catal10020214