Synthesis and Reactivity of Poly(propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes
Abstract
:1. Introduction
2. Results
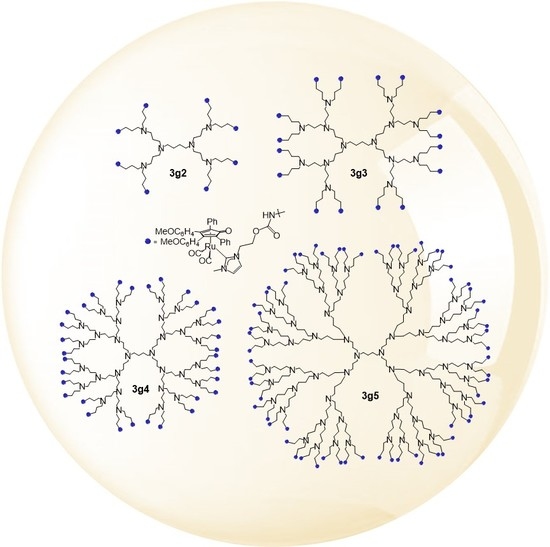
2.1. Synthesis and Characterization of the Ruthenium-Decorated Dendrimers
2.2. Catalytic Transfer Hydrogenation
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Dicarbonyl(η4-3,4-bis(4-methoxyphenyl)-2,5-diphenylcyclopenta-2,4-dienone) (1-methyl-3-(2-CO2Im-ethyl)imidazol-ylidene)ruthenium (2)
3.3. Synthesis of Functionalized Dendrimers 3g1–5
3.4. General Procedure for Transfer Hydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Astruc, D. Dendritic catalysis—Basic concepts and recent trends. Coord. Chem. Rev. 2013, 257, 2317–2334. [Google Scholar] [CrossRef]
- Astruc, D. Electron-transfer processes in dendrimers and their implication in biology, catalysis, sensing and nanotechnology. Nat. Chem. 2012, 4, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Méry, D.; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and Dendritic Catalysts. Acc. Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Oosterom, G.E.; Reek, J.N.H.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Transition Metal Catalysis Using Functionalized Dendrimers. Angew. Chem. Int. Ed. 2001, 40, 1828–1849. [Google Scholar] [CrossRef]
- Van Heerbeek, R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Dendrimers as Support for Recoverable Catalysts and Reagents. Chem. Rev. 2002, 102, 3717–3756. [Google Scholar] [CrossRef]
- Reek, J.N.H.; Arévalo, S.; van Heerbeek, R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Dendrimers in Catalysis. In Advances in Catalysis; Elsevier: San Diego, CA, USA, 2006; Volume 49, pp. 71–151. ISBN 978-0-12-007849-3. [Google Scholar]
- Dykes, G.M. Dendrimers: A review of their appeal and applications. J. Chem. Technol. Biotechnol. 2001, 76, 903–918. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Shreiner, C.D.; Moorefield, C.N.; Newkome, G.R. Recent progress and applications for metallodendrimers. New J. Chem. 2007, 31, 1192. [Google Scholar] [CrossRef]
- Twyman, L.J.; King, A.S.H.; Martin, I.K. Catalysis inside dendrimers. Chem. Soc. Rev. 2002, 31, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Baráth, E. Selective Reduction of Carbonyl Compounds via (Asymmetric) Transfer Hydrogenation on Heterogeneous Catalysts. Synthesis 2020, 52, 504–520. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. Dendritic effects: Dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J. Chem. 2012, 36, 264–281. [Google Scholar] [CrossRef]
- Helms, B.; Fréchet, J.M.J. The Dendrimer Effect in Homogeneous Catalysis. Adv. Synth. Catal. 2006, 348, 1125–1148. [Google Scholar] [CrossRef]
- Kakkar, A.K. Dendritic polymers: From efficient catalysis to drug delivery. Macromol. Symp. 2003, 196, 145–154. [Google Scholar] [CrossRef]
- Yi, B.; He, H.-P.; Fan, Q.-H. Synthesis and application of peripherally alkyl-functionalized dendritic pyrphos ligands: Homogeneous-supported catalysts for enantioselective hydrogenation. J. Mol. Catal. A Chem. 2010, 315, 82–85. [Google Scholar] [CrossRef]
- Yu, J.; RajanBabu, T.V.; Parquette, J.R. Conformationally Driven Asymmetric Induction of a Catalytic Dendrimer. J. Am. Chem. Soc. 2008, 130, 7845–7847. [Google Scholar] [CrossRef]
- Findeis, R.A.; Gade, L.H. Tripodal Phosphane Ligands with Novel Linker Units and Their Rhodium Complexes as Building Blocks for Dendrimer Catalysts. Eur. J. Inorg. Chem. 2003, 2003, 99–110. [Google Scholar] [CrossRef]
- Natarajan, B.; Jayaraman, N. Synthesis and studies of Rh (I) catalysts within and across poly(alkyl aryl ether) dendrimers. J. Organomet. Chem. 2011, 696, 722–730. [Google Scholar] [CrossRef]
- Rodríguez, L.I.; Rossell, O.; Seco, M.; Muller, G. Carbosilane dendrimers peripherally functionalized with P-stereogenic diphosphine ligands and related rhodium complexes. J. Organomet. Chem. 2009, 694, 1938–1942. [Google Scholar] [CrossRef]
- Rodríguez, L.-I.; Rossell, O.; Seco, M.; Muller, G. Rhodium or ruthenium units peripherally coordinated to carbosilane dendrimers functionalized with P-stereogenic monophosphines. J. Organomet. Chem. 2007, 692, 851–858. [Google Scholar] [CrossRef]
- Kassube, J.K.; Gade, L.H. Immobilisation of the Pyrphos Ligand on Soluble Hyperbranched Supports and Use in Rhodium-Catalysed Hydrogenation in Ionic Liquids. Adv. Synth. Catal. 2009, 351, 739–749. [Google Scholar] [CrossRef]
- Tang, W.-J.; Huang, Y.-Y.; He, Y.-M.; Fan, Q.-H. Dendritic MonoPhos: Synthesis and application in Rh-catalyzed asymmetric hydrogenation. Tetrahedron Asymm. 2006, 17, 536–543. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Li, Z.-W.; He, Y.-M.; Zhu, S.-F.; Fan, Q.-H.; Zhou, Q.-L. Modular chiral dendritic monodentate phosphoramidite ligands for Rh (II)-catalyzed asymmetric hydrogenation: Unprecedented enhancement of enantioselectivity. Chem. Commun. 2008, 45, 6048. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Deng, G.-J.; Li, Y.; He, Y.-M.; Tang, W.-J.; Fan, Q.-H. Enantioselective Hydrogenation of Quinolines Catalyzed by Ir (BINAP)-Cored Dendrimers: Dramatic Enhancement of Catalytic Activity. Org. Lett. 2007, 9, 1243–1246. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, T.-F.; Deng, J.-G.; Liu, H.; Cui, X.; Zhu, J.; Jiang, Y.-Z.; Choi, M.C.K.; Chan, A.S.C. Multiple Dendritic Catalysts for Asymmetric Transfer Hydrogenation. J. Org. Chem. 2002, 67, 5301–5306. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, T.-F.; Jiang, L.; Deng, J.-G.; Liu, H.; Zhu, J.; Jiang, Y.-Z. Synthesis of Dendritic Catalysts and Application in Asymmetric Transfer Hydrogenation. J. Org. Chem. 2005, 70, 1006–1010. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, T.-F.; Deng, J.-G.; Liu, H.; Jiang, Y.-Z.; Choi, M.C.K.; Chan, A.S.C. Dendritic catalysts for asymmetric transfer hydrogenation. Chem. Commun. 2001, 14, 1488–1489. [Google Scholar] [CrossRef]
- Liu, P.N.; Chen, Y.C.; Li, X.Q.; Tu, Y.Q.; Gen Deng, J. Dendritic catalysts for asymmetric transfer hydrogenation based (1S,2R)-norephedrine derived ligands. Tetrahedron Asymmetry 2003, 14, 2481–2485. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q. A fluorinated dendritic TsDPEN-Ru (ii) catalyst for asymmetric transfer hydrogenation of prochiral ketones in aqueous media. Chem. Commun. 2010, 46, 4616. [Google Scholar] [CrossRef] [PubMed]
- Cesari, C.; Mazzoni, R.; Müller-Bunz, H.; Albrecht, M. Ruthenium (0) complexes with triazolylidene spectator ligands: Oxidative activation for (de)hydrogenation catalysis. J. Organomet. Chem. 2015, 793, 256–262. [Google Scholar] [CrossRef]
- Cesari, C.; Cingolani, A.; Parise, C.; Zacchini, S.; Zanotti, V.; Cassani, M.C.; Mazzoni, R. Ruthenium hydroxycyclopentadienyl N-heterocyclic carbene complexes as transfer hydrogenation catalysts. RSC Adv. 2015, 5, 94707–94718. [Google Scholar] [CrossRef]
- Warner, M.C.; Casey, C.P.; Bäckvall, J.-E. Shvo’s Catalyst in Hydrogen Transfer Reactions. In Bifunctional Molecular Catalysis, Topics in Organometallic Chemistry; Ikariya, T., Shibasaki, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 37, pp. 85–125. ISBN 978-3-642-20730-3. [Google Scholar]
- Conley, B.L.; Pennington-Boggio, M.K.; Boz, E.; Williams, T.J. Discovery, Applications, and Catalytic Mechanisms of Shvo’s Catalyst. Chem. Rev. 2010, 110, 2294–2312. [Google Scholar] [CrossRef]
- Pasini, T.; Solinas, G.; Zanotti, V.; Albonetti, S.; Cavani, F.; Vaccari, A.; Mazzanti, A.; Ranieri, S.; Mazzoni, R. Substrate and product role in the Shvo’s catalyzed selective hydrogenation of the platform bio-based chemical 5-hydroxymethylfurfural. Dalton Trans. 2014, 43, 10224–10234. [Google Scholar] [CrossRef]
- Cesari, C.; Sambri, L.; Zacchini, S.; Zanotti, V.; Mazzoni, R. Microwave-Assisted Synthesis of Functionalized Shvo-Type Complexes. Organometallics 2014, 33, 2814–2819. [Google Scholar] [CrossRef]
- Cesari, C.; Conti, S.; Zacchini, S.; Zanotti, V.; Cassani, M.C.; Mazzoni, R. Sterically driven synthesis of ruthenium and ruthenium–silver N-heterocyclic carbene complexes. Dalton Trans. 2014, 43, 17240–17243. [Google Scholar] [CrossRef]
- Cingolani, A.; Cesari, C.; Zacchini, S.; Zanotti, V.; Cassani, M.C.; Mazzoni, R. Straightforward synthesis of iron cyclopentadienone N-heterocyclic carbene complexes. Dalton Trans. 2015, 44, 19063–19067. [Google Scholar] [CrossRef]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef] [PubMed]
- Schaper, L.-A.; Hock, S.J.; Herrmann, W.A.; Kühn, F.E. Synthesis and Application of Water-Soluble NHC Transition-Metal Complexes. Angew. Chem. Int. Ed. 2013, 52, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Crudden, C.M.; Allen, D.P. Stability and reactivity of N-heterocyclic carbene complexes. Coord. Chem. Rev. 2004, 248, 2247–2273. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly (N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef]
- Schuster, O.; Yang, L.; Raubenheimer, H.G.; Albrecht, M. Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other Classes of NHC Ligands with Reduced Heteroatom Stabilization. Chem. Rev. 2009, 109, 3445–3478. [Google Scholar] [CrossRef] [Green Version]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Nolan, S.P. (Ed.) N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2014; ISBN 978-3-527-33490-2. [Google Scholar]
- Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Fujihara, T.; Obora, Y.; Tokunaga, M.; Sato, H.; Tsuji, Y. Dendrimer N-heterocyclic carbene complexes with rhodium (I) at the core. Chem. Commun. 2005, 4526. [Google Scholar] [CrossRef] [PubMed]
- Virboul, M.A.N.; Lutz, M.; Siegler, M.A.; Spek, A.L.; van Koten, G.; Klein Gebbink, R.J.M. One-Pot Synthesis and Immobilisation of Sulfonate-Tethered N-Heterocyclic Carbene Complexes on Polycationic Dendrimers. Chem. Eur. J. 2009, 15, 9981–9986. [Google Scholar] [CrossRef] [PubMed]
- Anjali, J.K.; Sreekumar, K. Heterogeneous High-Loading Hyperbranched Polyglycidol with Peripheral NHC–Pd Complex: Synthesis and Application as Catalyst in Suzuki Coupling Reaction. Catal. Lett. 2019, 149, 1952–1964. [Google Scholar] [CrossRef]
- Busetto, L.; Cassani, M.C.; van Leeuwen, P.W.N.M.; Mazzoni, R. Synthesis of new poly(propylenimine) dendrimers DAB-dendr-[NH(O)COCH2CH2OC(O)C5H4Rh(NBD)]n {n = 4, 8, 16, 32, 64} functionalized with alkoxycarbonylcyclopentadienyl complexes of rhodium (I). Dalton Trans. 2004, 17, 2767–2770. [Google Scholar] [CrossRef]
- Cesari, C.; Mazzoni, R.; Matteucci, E.; Baschieri, A.; Sambri, L.; Mella, M.; Tagliabue, A.; Basile, F.L.; Lucarelli, C. Hydrogen Transfer Activation via Stabilization of Coordinatively Vacant Sites: Tuning Long-Range π-System Electronic Interaction between Ru(0) and NHC Pendants. Organometallics 2019, 38, 1041–1051. [Google Scholar] [CrossRef]





| Entry | [Ru] | Additive | Conversion (%) 8 h | Conversion (%) 24 h |
|---|---|---|---|---|
| 1 | 1a | CAN | 25 | 61 |
| 2 | 1b | CAN | 10 | 87 |
| 3 | 1c | CAN | 10 | 25 |

| Entry | [Ru] | Additive | Conversion (%) 8 h | Conversion (%) 24 h |
|---|---|---|---|---|
| 1 | 3g1 | CAN 2 | 9 | 17 |
| 2 | 3g2 | CAN 2 | 9 | 17 |
| 3 | 3g3 | CAN 2 | 7 | 15 |
| 4 | 3g4 | CAN 2 | 4 | 15 |
| 5 | 3g5 | CAN 2 | 6 | 16 |
| 6 | 3g1 | pyridine 3 | 0 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesari, C.; Conti, R.; Cingolani, A.; Zanotti, V.; Cassani, M.C.; Rigamonti, L.; Mazzoni, R. Synthesis and Reactivity of Poly(propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes. Catalysts 2020, 10, 264. https://doi.org/10.3390/catal10020264
Cesari C, Conti R, Cingolani A, Zanotti V, Cassani MC, Rigamonti L, Mazzoni R. Synthesis and Reactivity of Poly(propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes. Catalysts. 2020; 10(2):264. https://doi.org/10.3390/catal10020264
Chicago/Turabian StyleCesari, Cristiana, Riccardo Conti, Andrea Cingolani, Valerio Zanotti, Maria Cristina Cassani, Luca Rigamonti, and Rita Mazzoni. 2020. "Synthesis and Reactivity of Poly(propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes" Catalysts 10, no. 2: 264. https://doi.org/10.3390/catal10020264
APA StyleCesari, C., Conti, R., Cingolani, A., Zanotti, V., Cassani, M. C., Rigamonti, L., & Mazzoni, R. (2020). Synthesis and Reactivity of Poly(propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes. Catalysts, 10(2), 264. https://doi.org/10.3390/catal10020264