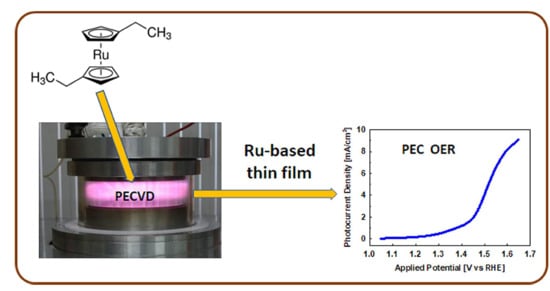
Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Over, H. Surface chemistry of ruthenium dioxide in heterogeneous catalysis and electrocatalysis: From fundamental to applied research. Chem. Rev. 2012, 112, 3356–3426. [Google Scholar] [CrossRef]
- Jeong, J.M.; Jin, S.B.; Yoon, J.H.; Yeo, J.G.; Lee, G.Y.; Irshad, M.; Lee, S.; Seo, D.; Kwak, B.E.; Choi, B.G.; et al. High-throughput production of heterogeneous RuO2/graphene catalyst in a hydrodynamic reactor for selective alcohol oxidation. Catalysts 2019, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wei, X.; Liu, C.; Liu, Y. The electrocatalytic application of RuO2 in direct borohydride fuel cells. Mater. Chem. Phys. 2014, 145, 269–273. [Google Scholar] [CrossRef]
- Takagi, Y.; Kerman, K.; Ko, C.; Ramanathan, S. Operational characteristics of thin film solid oxide fuel cells with ruthenium anode in natural gas. J. Power Sources 2013, 243, 1–9. [Google Scholar] [CrossRef]
- Tatsat, B.; Mukherjee, A. Overall water splitting under visible light irradiation using nanoparticulate RuO2 loaded Cu2O powder as photocatalyst. Energy Procedia 2014, 54, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Creus, J.; De Tovar, J.; Romero, N.; Garcia-Anton, J.; Philippot, K.; Bofill, R.; Sala, X. Ruthenium nanoparticles for catalytic water splitting. ChemSusChem 2019, 12, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Permana, A.D.C.; Kim, J.; Min, B.K. Water splitting for hydrogen production using a high surface area RuO2 electrocatalyst synthesized in supercritical water. Inter. J. Hydrog. Energy 2013, 38, 6092–6096. [Google Scholar] [CrossRef]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent advances and prospective in ruthenium-based materials for electrochemical water splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Li, H.; Cai, P.; Li, Y.; Wen, Z. Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting with 0.73 V in an asymmetric-electrolyte electrolyser. Nano Energy 2019, 61, 576–583. [Google Scholar] [CrossRef]
- Gómez-Solís, C.; Ballesteros, J.C.; Torres-Martínez, L.M.; Juárez-Ramírez, I. RuO2−NaTaO3 heterostructure for its application in photoelectrochemical water splitting under simulated sunlight illumination. Fuel 2016, 166, 36–41. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.W.; Ye, B.U.; Chun, S.H.; Joo, S.H.; Park, H.; Lee, H.; Jeong, H.Y.; Kim, M.H.; Baik, J.M. Structural evolution of chemically-driven RuO2 nanowires and 3-dimensional design for photo-catalytic applications. Sci. Rep. 2015, 5, 11933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Jirkovsky, J.; Hoffmannova, H.; Klementova, M.; Krtil, P.J. Particle size dependence of the electrocatalytic activity of nanocrystalline RuO2 electrodes. J. Electrochem. Soc. 2006, 153, E111–E118. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solution. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.; Liao, J.; Su, Y.; Xue, X.; Xing, W. Study of ruthenium oxide catalyst for electrocatalytic performance in oxygen evolution. J. Mol. Catal. A Chem. 2006, 247, 7–13. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.; Kim, H.Y.; Hwang, E.; Kim, H.J.; Ahn, S.H.; Kim, S.K. Activity and stability of the oxygen evolution reaction on electrodeposited Ru and its thermal oxides. Appl. Surf. Sci. 2015, 359, 227–235. [Google Scholar] [CrossRef]
- Han, J.; An, H.J.; Kim, T.W.; Lee, K.Y.; Kim, H.J.; Kim, Y.; Chae, H.J. Effect of structure-controlled ruthenium oxide by nanocasting in electrocatalytic oxygen and chlorine evolution reactions in acidic conditions. Catalysts 2019, 9, 549. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, E.; Imanishi, A.; Fukui, K.; Nakato, Y. Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution. Electrochim. Acta 2011, 56, 2009–2016. [Google Scholar] [CrossRef]
- Das, S.K.; Dutta, P.K. Synthesis and characterization of a ruthenium oxide–zeolite Y catalyst for photochemical oxidation of water to dioxygen. Micropor. Mesopor. Mat. 1998, 22, 475–483. [Google Scholar] [CrossRef]
- Mamaca, N.; Mayousse, E.; Arrii-Clacens, S.; Napporn, T.W.; Servat, K.; Guillet, N.; Kokoh, K.B. Electrochemical activity of ruthenium and iridium based catalysts for oxygen evolution reaction. Appl. Catal. B Environ. 2012, 111–112, 376–380. [Google Scholar] [CrossRef]
- Yi, J.; Lee, W.H.; Choi, C.H.; Lee, Y.; Park, K.S.; Min, B.K.; Hwang, Y.J.; Oh, H.S. Effect of Pt introduced on Ru-based electrocatalyst for oxygen evolution activity and stability. Electrochem. Commun. 2019, 104, 106469. [Google Scholar] [CrossRef]
- Azevedo, J.; Steier, L.; Dias, P.; Stefik, M.; Sousa, C.T.; Araújo, J.P.; Mendes, A.; Graetzel, M.; Tilley, S.D. On the stability enhancement of cuprous oxide water splitting photocathodes by low temperature steam annealing. Energy Environ. Sci. 2014, 7, 4044–4052. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Zhu, J.; Zhang, M.; Hong, Q.; Han, J.; Liu, J. Synergistic photoelectrochemical performance of La-doped RuO2-TiO2/Ti electrodes. Appl. Surf. Sci. 2020, 502, 144288. [Google Scholar] [CrossRef]
- Tyczkowski, J. Cold plasma—a promising tool for the development of electrochemical cells. In Electrochemical Cells—New Advances in Fundamental Researches and Applications; Shao, Y., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 5; pp. 105–138. [Google Scholar] [CrossRef]
- Tyczkowski, J. Cold plasma produced catalytic materials. In Plasma Science and Technology—Progress in Physical States and Chemical Reactions; Mieno, T., Ed.; InTech: Rijeka, Croatia, 2016; Chapter 3; pp. 25–65. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tracz, P.; Redzynia, W.; Tyczkowski, J. Plasma deposited novel nanocatalysts for CO2 hydrogenation to methane. J. CO2 Util. 2017, 17, 312–319. [Google Scholar] [CrossRef]
- Li, L.; Tian, T.; Jiang, J.; Ai, L. Hierarchically porous Co3O4 architectures for efficient oxygen generation from electrochemical water splitting. J. Power Sources 2015, 294, 103–111. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolution reaction and oxygen evolution reaction electrocatalysts for solar water splitting devices. JACS 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [Green Version]
- Balcerzak, J.; Redzynia, W.; Tyczkowski, J. In-situ XPS analysis of oxidized and reduced plasma deposited ruthenium-based thin catalytic films. Appl. Surf. Sci. 2017, 426, 852–855. [Google Scholar] [CrossRef]
- Kaur, M.; Chhetri, M.; Rao, C.N.R. Photoelectrochemical OER activity by employing BiVO4 with manganese oxide co-catalysts. Phys. Chem. Chem. Phys. 2020, 22, 811–817. [Google Scholar] [CrossRef]
- Doyle, R.L.; Godwin, I.J.; Brandon, M.P.; Lyons, M.E.G. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15, 13737–13783. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Rao, R.R.; Wang, X.R.; Hong, W.T.; Rouleau, M.; Shao-Horn, Y. The role of Ru redox in pH-dependent oxygen evolution on rutile ruthenium dioxide surfaces. Chem 2017, 2, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Stoerzinger, K.A.; Diaz-Morales, O.; Kolb, M.; Rao, R.R.; Frydendal, R.; Qiao, L.; Wang, X.R.; Halck, N.B.; Rossmeisl, J.; Hansen, H.A.; et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2017, 2, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.C.; Gouveia, A.F.; Sczancoski, J.C.; Santos, R.S.; Sá, J.L.S.; Longo, E.; Cavalcante, L.S. Electronic structure, morphological aspects, optical and electrochemical properties of RuO2 nanocrystals. Electron. Mater. Lett. 2019, 15, 645–653. [Google Scholar] [CrossRef]
- Tong, K.Y.; Jelenkovic, V.; Cheung, W.Y.; Wong, S.P. Temperature dependence of resistance in reactively sputtered RuO2 thin films. J. Mater. Sci. Lett. 2001, 20, 699–700. [Google Scholar] [CrossRef]
- Steeves, M.M.; Lad, R.J. Influence of nanostructure on charge transport in RuO2 thin films. J. Vac. Sci. Technol. A 2010, 28, 906–911. [Google Scholar] [CrossRef]
- Alberding, B.G.; DeSario, P.A.; So, C.R.; Dunkelberger, A.D.; Rolison, D.R.; Owrutsky, J.C.; Heilweil, E.J. Static and time-resolved terahertz measurements of photoconductivity in solution-deposited ruthenium dioxide nanofilms. J. Phys. Chem. C 2017, 121, 4037–4044. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, E.; Trasatti, S. Electrocatalysis in water electrolysis. In Catalysis for Sustainable Energy Production; Barbaro, P., Bianchini, C., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2009; Chapter 7.5; pp. 255–264. [Google Scholar]
- Patil, P.S.; Ennaoui, E.A.; Lokhande, C.D.; Müller, M.; Giersing, M.; Diesner, K.; Tributsch, H. Characterization of ultrasonic spray pyrolysed ruthenium oxide thin films. Thin Solid Film. 1997, 310, 57–62. [Google Scholar] [CrossRef]
- Kao, K.C.; Hwang, W. Electrical Transport in Solids; Pergamon Press: Oxford, UK, 1981; Chapter 6.3; pp. 436–453. [Google Scholar]
- Simonov, P.A.; Likholobov, V.A. Physicochemical aspects of preparation of carbon-supported noble metal catalysts. In Catalysis and Electrocatalysis at Nanoparticle Surfaces; Wieckowski, A., Savinova, E.R., Vayenas, C.G., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Chapter 12.1. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kityk, I.V.; Nagao, T. Synthesis, structural, and electrical characterization of RuO2 sol–gel spin-coating nano-films. J. Mater. Sci. Mater. Electron. 2016, 27, 10791–10797. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozwiak, L.; Balcerzak, J.; Tyczkowski, J. Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting. Catalysts 2020, 10, 278. https://doi.org/10.3390/catal10030278
Jozwiak L, Balcerzak J, Tyczkowski J. Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting. Catalysts. 2020; 10(3):278. https://doi.org/10.3390/catal10030278
Chicago/Turabian StyleJozwiak, Lukasz, Jacek Balcerzak, and Jacek Tyczkowski. 2020. "Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting" Catalysts 10, no. 3: 278. https://doi.org/10.3390/catal10030278
APA StyleJozwiak, L., Balcerzak, J., & Tyczkowski, J. (2020). Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting. Catalysts, 10(3), 278. https://doi.org/10.3390/catal10030278