Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Synthesis
2.2. Material Analysis
2.3. Morphological Analysis
2.4. Optical Properties
2.5. Surface Analysis
2.6. Photoelectrochemical (PEC) Measurements
2.7. Photocatalytic Activity Tests for H2 Generation from Water
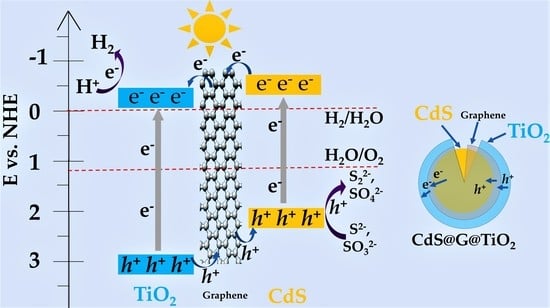
2.8. Reaction Mechanism
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of CdS Nanoparticles
3.3. Synthesis of Graphene Quantum Dots (GQD)
3.4. Synthesis of CdS@Graphene@TiO2 Samples (CdS@G@TiO2)
3.5. Material Characterization Techniques
3.6. Photoelectrochemical (PEC) Measurements
3.7. Photocatalytic H2 Generation Setup
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ul’yanin, Y.A.; Kharitonov, V.V.; Yurshina, D.Y. Forecasting the Dynamics of the Depletion of Conventional Energy Resources. Stud. Russ. Econ. Dev. 2018, 29, 153–160. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.-W.; Lee, J.S. Toward practical solar hydrogen production—An artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar Light-Responsive Pt/CdS/TiO2 Photocatalysts for Hydrogen Production and Simultaneous Degradation of Inorganic or Organic Sacrificial Agents in Wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Xu, R. Photochemical Deposition of Pt on CdS for H2 Evolution from Water: Markedly Enhanced Activity by Controlling Pt Reduction Environment. J. Phys. Chem. C 2013, 117, 783–790. [Google Scholar] [CrossRef]
- Liu, M.; Du, Y.; Ma, L.; Jing, D.; Guo, L. Manganese doped cadmium sulfide nanocrystal for hydrogen production from water under visible light. Int. J. Hydrog. Energy 2012, 37, 730–736. [Google Scholar] [CrossRef]
- Oskoui, M.S.; Khatamian, M.; Haghighi, M.; Yavari, A. Photocatalytic hydrogen evolution from water over chromosilicate-based catalysts. RSC Adv. 2014, 4, 19569–19577. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.K.; Lim, S.K.; Kim, S.; In, S.-I. Efficient visible light-induced H2 production by Au@CdS/TiO2 nanofibers: Synergistic effect of core–shell structured Au@CdS and densely packed TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 166–167, 423–431. [Google Scholar] [CrossRef]
- Fang, J.; Xu, L.; Zhang, Z.; Yuan, Y.; Cao, S.; Wang, Z.; Yin, L.; Liao, Y.; Xue, C. Au@TiO2–CdS Ternary Nanostructures for Efficient Visible-Light-Driven Hydrogen Generation. ACS Appl. Mater. Interfaces 2013, 5, 8088–8092. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Gao, D.; Wang, P.; Tang, H. Improved H2-generation performance of Pt/CdS photocatalyst by a dual-function TiO2 mediator for effective electron transfer and hole blocking. Ceram. Int. 2019, 45, 9807–9813. [Google Scholar] [CrossRef]
- Li, A.; Zhu, W.; Li, C.; Wang, T.; Gong, J. Rational design of yolk–shell nanostructures for photocatalysis. Chem. Soc. Rev. 2019, 48, 1874–1907. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Synthesis of One-Dimensional CdS@TiO2 Core–Shell Nanocomposites Photocatalyst for Selective Redox: The Dual Role of TiO2 Shell. ACS Appl. Mater. Interfaces 2012, 4, 6378–6385. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Pan, F.; Xu, L.; Zheng, M.; Sow, C.H.; Wu, K.; Xu, G.Q.; Chen, W. Facile synthesis of CdS@TiO2 core–shell nanorods with controllable shell thickness and enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2015, 349, 279–286. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, X.; Li, X.; Zhang, D.; Zhu, J.; Li, G. Core-shell structure CdS/TiO2 for enhanced visible-light-driven photocatalytic organic pollutants degradation. J. Sol-Gel Sci. Technol. 2013, 66, 504–511. [Google Scholar] [CrossRef]
- Zubair, M.; Svenum, I.-H.; Rønning, M.; Yang, J. Facile synthesis approach for core-shell TiO2–CdS nanoparticles for enhanced photocatalytic H2 generation from water. Catal. Today 2019, 328, 15–20. [Google Scholar] [CrossRef]
- El-Atab, N.; Chowdhury, F.; Ulusoy, T.G.; Ghobadi, A.; Nazirzadeh, A.; Okyay, A.K.; Nayfeh, A. ~3-nm ZnO Nanoislands Deposition and Application in Charge Trapping Memory Grown by Single ALD Step. Sci. Rep. 2016, 6, 38712. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A. Recent progress in magnetic iron oxide–semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ren, F.; Zhang, S.; Wu, W.; Xiao, X.; Liu, Y.; Jiang, C. SiO2–Ag–SiO2–TiO2 multi-shell structures: Plasmon enhanced photocatalysts with wide-spectral-response. J. Mater. Chem. A 2013, 1, 13128–13138. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible Light-Driven α-Fe2O3 Nanorod/Graphene/BiV1−xMoxO4 Core/Shell Heterojunction Array for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic process in TiO2/graphene hybrid materials. Evidence of charge separation by electron transfer from reduced graphene oxide to TiO2. Catal. Today 2017, 281, 29–37. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J. Graphene-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2013, 4, 753–759. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.; Razzaq, A.; Grimes, C.A.; In, S.-I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded with graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Kuznetsova, Y.V.; Rempel, A.A. Size and zeta potential of CdS nanoparticles in stable aqueous solution of EDTA and NaCl. Inorg. Mater. 2015, 51, 215–219. [Google Scholar] [CrossRef]
- Park, S.W.; Huang, C.P. The surface acidity of hydrous CdS(s). J. Colloid Interface Sci. 1987, 117, 431–441. [Google Scholar] [CrossRef]
- Liu, S.; Yang, M.-Q.; Xu, Y.-J. Surface charge promotes the synthesis of large, flat structured graphene–(CdS nanowire)–TiO2 nanocomposites as versatile visible light photocatalysts. J. Mater. Chem. A 2014, 2, 430–440. [Google Scholar] [CrossRef]
- Liu, H.; Lv, T.; Wu, X.; Zhu, C.; Zhu, Z. Preparation and enhanced photocatalytic activity of CdS@RGO core–shell structural microspheres. Appl. Surf. Sci. 2014, 305, 242–246. [Google Scholar] [CrossRef]
- Kuai, L.; Zhou, Y.; Tu, W.; Li, P.; Li, H.; Xu, Q.; Tang, L.; Wang, X.; Xiao, M.; Zou, Z. Rational construction of a CdS/reduced graphene oxide/TiO2 core–shell nanostructure as an all-solid-state Z-scheme system for CO2 photoreduction into solar fuels. RSC Adv. 2015, 5, 88409–88413. [Google Scholar] [CrossRef]
- Chen, G.; Wu, S.; Hui, L.; Zhao, Y.; Ye, J.; Tan, Z.; Zeng, W.; Tao, Z.; Yang, L.; Zhu, Y. Assembling carbon quantum dots to a layered carbon for high-density supercapacitor electrodes. Sci. Rep. 2016, 6, 19028. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Q.-H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.-X.; Wang, M.; Cheng, H.-M. Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Haque, S.E.; Ramdas, B.; Sheela, A.; Padmavathy, N. Facile one-pot low-temperature solid-state approach towards phase transformation of nanoCdS. Micro Nano Lett. 2014, 9, 731–735. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Barhoum, A.; Nada, A.A.; Moustafa, Y.M.; Seliman, S.M.; Youssef, A.M.; Bechelany, M. Synthesis of mesoporous core-shell CdS@TiO2 (0D and 1D) photocatalysts for solar-driven hydrogen fuel production. J. Photochem. Photobiol. A Chem. 2018, 351, 261–270. [Google Scholar] [CrossRef]
- Ge, M.-Z.; Li, S.-H.; Huang, J.-Y.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 nanotube arrays loaded with reduced graphene oxide films: Facile hybridization and promising photocatalytic application. J. Mater. Chem. A 2015, 3, 3491–3499. [Google Scholar] [CrossRef]
- Liang, Y.; Zhai, L.; Zhao, X.; Xu, D. Band-Gap Engineering of Semiconductor Nanowires through Composition Modulation. J. Phys. Chem. B 2005, 109, 7120–7123. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, C.; Hou, J.; Wang, F.; Min, S.; Ma, X.; Jin, Z.; Xu, J.; Lu, G.; Huang, K.-W. Strongly coupled CdS/graphene quantum dots nanohybrids for highly efficient photocatalytic hydrogen evolution: Unraveling the essential roles of graphene quantum dots. Appl. Catal. B Environ. 2017, 216, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Gharedaghi, S.; Kimiagar, S.; Safa, S. P-Nitrophenol Degradation Using N-Doped Reduced Graphene-CdS Nanocomposites. Phys. Status Solidi 2018, 215, 1700618. [Google Scholar] [CrossRef]
- Yang, H.B.; Dong, Y.Q.; Wang, X.; Khoo, S.Y.; Liu, B. Cesium Carbonate Functionalized Graphene Quantum Dots as Stable Electron-Selective Layer for Improvement of Inverted Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 1092–1099. [Google Scholar] [CrossRef]
- He, G.; Zhang, Y.; He, Q. MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light. Catalysts 2019, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, X.; Meng, S.; Jiang, X.; Wang, J.; Chen, S. Ultra-low content of Pt modified CdS nanorods: One-pot synthesis and high photocatalytic activity for H2 production under visible light. J. Mater. Chem. A 2015, 3, 23732–23742. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl. Catal. B Environ. 2013, 138–139, 299–303. [Google Scholar] [CrossRef]
- Duta, A.; Visa, M.; Manolache, S.A.; Nanu, M. Anatase (TiO2) Thin Layers for Solar Energy Conversion. In Proceedings of the 2008 11th International Conference on Optimization of Electrical and Electronic Equipment, Brasov, Romania, 22–24 May 2008; pp. 461–466. [Google Scholar]
- Wei, H.H.-Y.; Evans, C.M.; Swartz, B.D.; Neukirch, A.J.; Young, J.; Prezhdo, O.V.; Krauss, T.D. Colloidal Semiconductor Quantum Dots with Tunable Surface Composition. Nano Lett. 2012, 12, 4465–4471. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Asanov, I.P.; Larionov, S.V.; Kudashov, A.G.; Leonova, T.G.; Bulusheva, L.G. Growth of CdS nanoparticles on the aligned carbon nanotubes. Phys. Chem. Chem. Phys. 2010, 12, 10871–10875. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.-J. Fabrication of coenocytic Pd@CdS nanocomposite as a visible light photocatalyst for selective transformation under mild conditions. J. Mater. Chem. 2012, 22, 5042–5052. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.C.; Fu, X. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. J. Mol. Catal. A Chem. 2006, 244, 25–32. [Google Scholar] [CrossRef]
- Jimmy, C.Y.; Yu, J.; Tang, H.Y.; Zhang, L. Effect of surface microstructure on the photoinduced hydrophilicity of porous TiO2 thin films. J. Mater. Chem. 2002, 12, 81–85. [Google Scholar]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon N. Y. 2015, 82, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Murashkina; Bakiev; Artemev; Rudakova; Emeline; Bahnemann Photoelectrochemical Behavior of the Ternary Heterostructured Systems CdS/WO3/TiO2. Catalysts 2019, 9, 999. [CrossRef] [Green Version]
- Kumar, R.; El-Shishtawy, R.; Barakat, M. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Santoni, A.; Biccari, F.; Malerba, C.; Valentini, M.; Chierchia, R.; Mittiga, A. Valence band offset at the CdS/Cu2ZnSnS4 interface probed by x-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 2013, 46, 175101. [Google Scholar] [CrossRef]
- Dias, S.; Krupanidhi, S.B. Determination of band offsets at the Al: ZnO/Cu2SnS3 interface using X-ray photoelectron spectroscopy. AIP Adv. 2015, 5, 047137. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Zheng, P.; Senty, T.; Meng, F.; Bristow, A.D.; Manivannan, A.; Wu, N. Solar Hydrogen Generation by a CdS-Au-TiO 2 Sandwich Nanorod Array Enhanced with Au Nanoparticle as Electron Relay and Plasmonic Photosensitizer. J. Am. Chem. Soc. 2014, 136, 8438–8449. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon N. Y. 2012, 50, 4738–4743. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Svenum, I.-H.; Rønning, M.; Yang, J. Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation. Catalysts 2020, 10, 358. https://doi.org/10.3390/catal10040358
Zubair M, Svenum I-H, Rønning M, Yang J. Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation. Catalysts. 2020; 10(4):358. https://doi.org/10.3390/catal10040358
Chicago/Turabian StyleZubair, Muhammad, Ingeborg-Helene Svenum, Magnus Rønning, and Jia Yang. 2020. "Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation" Catalysts 10, no. 4: 358. https://doi.org/10.3390/catal10040358
APA StyleZubair, M., Svenum, I. -H., Rønning, M., & Yang, J. (2020). Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation. Catalysts, 10(4), 358. https://doi.org/10.3390/catal10040358