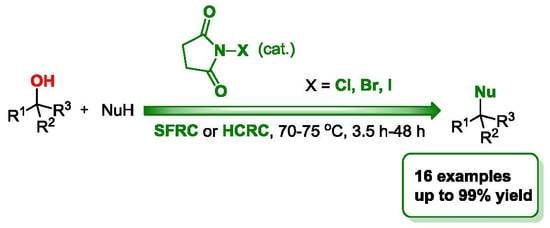
N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Yasuda, M.; Saito, T.; Ueba, M.; Baba, A. Direct Substitution of the Hydroxy Group in Alcohols with Silyl Nucleophiles Catalyzed by Indium Trichloride. Angew. Chem. Int. Ed. 2004, 43, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P.G. Direct Nucleophilic SN1-Type Reactions of Alcohols. Eur. J. Org. Chem. 2011, 2011, 647–666. [Google Scholar] [CrossRef]
- Kumar, R.; Van der Eycken, E.V. Recent approaches for C-C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 2013, 42, 1121–1146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; He, T.; Ma, L.; Wang, Z. Recent developments in Ritter reaction. RSC Advances 2014, 4, 64936–64946. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.-P.; Wang, C.-H.; Zhou, J. Catalytic functionalization of tertiary alcohols to fully substituted carbon centres. Org. Biomol. Chem. 2014, 12, 6033–6048. [Google Scholar] [CrossRef]
- Uchuskin, M.G.; Makarov, A.S.; Butin, A.V. Catalytic Alkylation of Furans by π-Activated Alcohols (Review). Chem. Heterocycl. Compd. 2014, 50, 791–806. [Google Scholar] [CrossRef]
- Chaskar, A.; Murugan, K. Direct allylation of alcohols using allyltrimethylsilane: A move towards an economical and ecological protocol for C-C bond formation. Catal. Sci. Tech. 2014, 4, 1852–1868. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Z.-Q. Iron-Catalyzed Alkylation of Alkenes and Alkynes Using Alcohols as the Alkylating Reagent. Synthesis 2015, 47, 1706–1708. [Google Scholar] [CrossRef]
- Dryzhakov, M.; Richmond, E.; Moran, J. Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols. Synthesis 2016, 48, 935–959. [Google Scholar]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, part ii, 288–329. [Google Scholar] [CrossRef] [Green Version]
- Guillena, G.; Ramón, D.J.; Yus, M. Alcohols as Electrophiles in C–C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angew. Chem. Int. Ed. 2007, 46, 2358–2364. [Google Scholar] [CrossRef]
- Dobereiner, G.E.; Crabtree, R.H. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. [Google Scholar] [CrossRef]
- Yang, G.-P.; Jiang, N.; Huang, X.-Q.; Yu, B.; Hu, C.-W. Non-corrosive heteropolyacid-based recyclable ionic liquid catalyzed direct dehydrative coupling of alcohols with alcohols or alkenes. Mol. Catal. 2019, 468, 80–85. [Google Scholar] [CrossRef]
- Chevella, D.; Macharla, A.K.; Kodumuri, S.; Banothu, R.; Gajula, K.S.; Amrutham, V.; Gennadievna, G.E.N.; Nama, N. Synthesis of internal olefins by direct coupling of alcohols and olefins over Moβ zeolite. Catal. Commun. 2019, 123, 114–118. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bora, U. Molecular Iodine-Catalyzed Selective C-3 Benzylation of Indoles with Benzylic Alcohols: A Greener Approach toward Benzylated Indoles. ACS Omega 2019, 4, 11770–11776. [Google Scholar] [CrossRef] [PubMed]
- Veenboer, R.M.P.; Nolan, S.P. Gold(i)-catalysed dehydrative formation of ethers from benzylic alcohols and phenols. Green Chem. 2015, 17, 3819–3825. [Google Scholar] [CrossRef] [Green Version]
- Böldl, M.; Fleischer, I. Dehydrative Coupling of Benzylic Alcohols Catalyzed by Brønsted Acid/Lewis Base. Eur. J. Org. Chem. 2019, 2019, 5856–5861. [Google Scholar] [CrossRef] [Green Version]
- Kolvari, E.; Ghorbani-Choghamarani, A.; Salehi, P.; Shirini, F.; Zolfigol, M.A. Application of N-halo reagents in organic synthesis. J. Iran Chem. Soc. 2007, 4, 126–174. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules 2016, 21, 1325. [Google Scholar] [CrossRef] [Green Version]
- Karimi, B.; Ebrahimian, G.R.; Seradj, H. Efficient and Chemoselective Conversion of Carbonyl Compounds to 1,3-Dioxanes Catalyzed with N-Bromosuccinimide under Almost Neutral Reaction Conditions. Org. Lett. 1999, 1, 1737–1739. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a Novel and Highly Effective Catalyst for Acetylation of Alcohols under Mild Reaction Conditions. Synlett 2001, 2001, 0519–0520. [Google Scholar] [CrossRef]
- Čebular, K.; Božić, B.Đ.; Stavber, S. Esterification of Aryl/Alkyl Acids Catalysed by N-bromosuccinimide under Mild Reaction Conditions. Molecules 2018, 23, 2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagh, Y.S.; Sawant, D.N.; Bhanage, B.M. Metal-free N-iodosuccinimide-catalyzed mild oxidative C–H bond amination of benzoxazoles. Tetrahedron Lett. 2012, 53, 3482–3485. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. N-Iodosuccinimide (NIS) a novel and effective catalyst for the cyanosilylation of aldehydes under mild reaction conditions. Catal. Commun. 2008, 9, 1342–1345. [Google Scholar] [CrossRef]
- Guha, S.; Rajeshkumar, V.; Kotha, S.S.; Sekar, G. A Versatile and One-Pot Strategy to Synthesize α-Amino Ketones from Benzylic Secondary Alcohols Using N-Bromosuccinimide. Org. Lett. 2015, 17, 406–409. [Google Scholar] [CrossRef]
- Muneeswara, M.; Muthukumar, A.; Sekar, G. Dual Role of N-Bromosuccinimide as Oxidant and Succinimide Surrogate in Domino One-Pot Oxidative Amination of Benzyl Alcohols for the Synthesis of α–Imido Ketones. ChemistrySelect 2018, 3, 12524–12529. [Google Scholar] [CrossRef]
- Muneeswara, M.; Sundaravelu, N.; Sekar, G. NBS-mediated synthesis of β-keto sulfones from benzyl alcohols and sodium arenesulfinates. Tetrahedron 2019, 75, 3479–3484. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic Oxidative Iodination of Organic Compounds with Iodide Catalyzed by Sodium Nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards Greener Fluorine Organic Chemistry: Direct Electrophilic Fluorination of Carbonyl Compounds in Water and Under Solvent-Free Reaction Conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Chun, S.; Chung, Y.K. Silver/NBS-Catalyzed Synthesis of α-Alkylated Aryl Ketones from Internal Alkynes and Benzyl Alcohols via Ether Intermediates. Org. Lett. 2018, 20, 5583–5586. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Breugst, M.; von der Heiden, D. Mechanisms in Iodine Catalysis. Chem. Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2006, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Čebular, K.; Božić, B.Đ.; Stavber, S. 1,3-Dibromo-5,5-dimethylhydantoin as a Precatalyst for Activation of Carbonyl Functionality. Molecules 2019, 24, 2608. [Google Scholar] [CrossRef] [Green Version]
- Beebe, T.R.; Adkins, R.L.; Bogardus, C.C.; Champney, B.; Hii, P.S.; Reinking, P.; Shadday, J.; Weatherford, W.D.; Webb, M.W.; Yates, S.W. Primary alcohol oxidation with N-iodosuccinimide. J. Org. Chem. 1983, 48, 3126–3128. [Google Scholar] [CrossRef]
- Anxionnat, B.; Guérinot, A.; Reymond, S.; Cossy, J. FeCl3-catalyzed Ritter reaction. Synthesis of amides. Tetrahedron Lett. 2009, 50, 3470–3473. [Google Scholar] [CrossRef]
- Theerthagiri, P.; Lalitha, A.; Arunachalam, P.N. Iodine-catalyzed one-pot synthesis of amides from nitriles via Ritter reaction. Tetrahedron Lett. 2010, 51, 2813–2819. [Google Scholar] [CrossRef]
- Ogata, O.; Nara, H.; Fujiwhara, M.; Matsumura, K.; Kayaki, Y. N-Monomethylation of Aromatic Amines with Methanol via PNHP-Pincer Ru Catalysts. Org. Lett. 2018, 20, 3866–3870. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Direct halogenation of alcohols with halosilanes under catalyst- and organic solvent-free reaction conditions. Tetrahedron Lett. 2016, 57, 2430–2433. [Google Scholar] [CrossRef]





| Entry | NXS | Mol % | Reaction Conditions | Conversion b (%) of 1 | Relative Distribution b (%) | |
|---|---|---|---|---|---|---|
| 3 | 4 | |||||
| 1 | - | - | MeOH (1mmol), 70–75 °C, 6 h | - | - | - |
| 2 | NIS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 100 | - |
| 3 | NCS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 92 | 5 c |
| 4 | NBS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 92 | 5 c |
| 5 | NIS | 2 | 70–75 °C, 3.5 h | 100 | - | 100 |
| 6 | NIS | 2 | In the dark, MeOH (1 mmol), 70–75 °C, 6 h | 100 | 100 | - |
| 7 | NIS | 2 | TEMPO (10 mol %), MeOH (1 mmol), 70–75 °C, 6 h | 100 | 100 | - |

| Entry | NXS | Mol % | Conversion b (%) of 1 | Relative Distribution b (%) | |
|---|---|---|---|---|---|
| 8 | 4 | ||||
| 1 | - | - | 0 | - | - |
| 2 | NCS | 3 | - | - | - |
| 3 | NBS | 3 | 4 | - | 4 |
| 4 | NIS | 3 | 100 | 100 | - |
| 5 | NIS | 1 | 100 | 100 | - |
| 6 c | NIS | 1 | 100 | 100 | - |
| 7 d | NIS | 1 | 100 | 100 | |

| Entry | NXS | Mol % | Conversion b (%) of 14 | Relative Distribution b (%) | ||
|---|---|---|---|---|---|---|
| 16 | 17 | 18 | ||||
| 1 | - | - | - | - | - | - |
| 2 | NBS | 10 | 78 | 67 | - | 11 |
| 3 | NCS | 10 | 82 | 72 | - | 10 |
| 4 | NIS | 10 | 84 | 80 | 2 | 2 |
| 5 c | NIS | 10 | 84 | 80 | 2 | 2 |
| 6 d | NIS | 10 | 84 | 80 | 2 | 2 |

| Entry | Bond Formation | R1, R2, R3 | NuY | Product | Conversion.b (%) (Yield c (%)) |
|---|---|---|---|---|---|
| 1 | C–O | R1 = R2 = Me R3 = (CH2)2Ph 5 | MeOH 2 |  | 74 (64) |
| 2 | R1 = Ph, R1 = R2 = Me 33 | MeOH 2 |  | 93 (90) | |
| 3 | R1 = Ph, R2 = H, R3 = Me 11 | MeOH 2 |  | 67 f (61) g | |
| 4 | R1 = Ph, R2 = R3 = H 27 | MeOH 2 |  | 2 f - | |
| 5 | R1 = 4-MePh, R2 = H, R3 = Ph 30 | TMSOEt 23c |  | 100 f (89) | |
| 6 | C–C | R1 = R2 = R3 = Ph 36 |  |  | - |
| 7 | R1 = Ph, R2 = R3 = H 27 |  |  | - | |
| 8 | R1 = R2 = Ph, R3 = H 1 |  |  | 100 e (92) | |
| 9 | C–N | R1 = Ph, R2 = R3 = H 27 | MeCN/H2O 15 |  | 8 f - |
| 10 | R1 = R2 = R3 = Ph 36 | MeCN/H2O 15 |  | - | |
| 11 | R1 = R2 = Ph, R3 = H 1 |  |  | 85 f (79) | |
| 12 | R1 = R2 = Ph, R3 = H 1 | MeCN/H2O 15 |  | 100 f (90) | |
| 13 | R1=Ph, R2=H, R3=Me 11 | MeCN/H2O 15 |  | 100 f (93) | |
| 14 | R1 = 4-MePh, R2 = H, R3 = Ph 30 | TMSNCS 23b |  | 100 (97) | |
| 15h | C–Cl | R1 = 3-NO2Ph, R2 = R3 = H 24 | TMSCl 23a |  | [42] |
| 16 | R1 = 3-NO2Ph, R2 = R3 = H 24 | TMSCl 23a |  | 91 d (76) | |
| 17h | R1 = Ph, R2 = R3 = H 27 | TMSCl 23a |  | 69 (65) [42] | |
| 18 | R1 = Ph, R2 = R3 = H 27 | TMSCl 23a |  | 100 d (88) | |
| 19h | R1 = 4-MePh, R2 = H, R3 = Me 14 | TMSCl 23a |  | 90 (86) [42] | |
| 20 | R1 = 4-MePh, R2 = H, R3 = Me 14 | TMSCl 23a |  | 100 (98) | |
| 21h | R1=Ph, R1=R2=Me, 33 | TMSCl 23a |  | 100 (98) [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Stavber, S. N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts 2020, 10, 460. https://doi.org/10.3390/catal10040460
Ajvazi N, Stavber S. N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts. 2020; 10(4):460. https://doi.org/10.3390/catal10040460
Chicago/Turabian StyleAjvazi, Njomza, and Stojan Stavber. 2020. "N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions" Catalysts 10, no. 4: 460. https://doi.org/10.3390/catal10040460
APA StyleAjvazi, N., & Stavber, S. (2020). N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts, 10(4), 460. https://doi.org/10.3390/catal10040460