Higher Activity of Ni/γ-Al2O3 over Fe/γ-Al2O3 and Ru/γ-Al2O3 for Catalytic Ammonia Synthesis in Nonthermal Atmospheric-Pressure Plasma of N2 and H2
Abstract
:1. Introduction
2. Results and Discussion
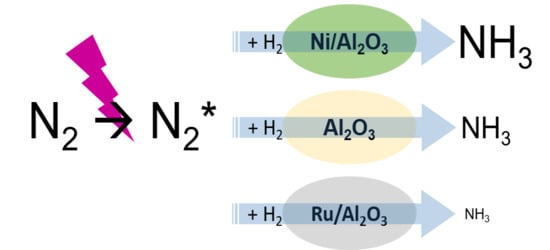
2.1. Comparison of the Activities of Various Oxide Catalysts
2.2. Activity and Active Sites of Ni/Al2O3 for Ammonia Synthesis
2.3. Kinetic Analysis of Ammonia Synthesis on Ni/Al2O3
3. Materials and Methods
3.1. Experimental Methods
3.2. Materials
3.3. Characterization of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hara, M.; Kitano, M.; Hosono, H. Ru-Loaded C12A7:e– Electride as a Catalyst for Ammonia Synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Abe, H.; Niwa, Y.; Kitano, M.; Inoue, Y.; Sasase, M.; Nakao, T.; Tada, T.; Yokoyama, T.; Hara, M.; Hosono, H. Anchoring Bond Between Ru and N Atoms of Ru/Ca2NH Catalyst: Crucial for the High Ammonia Synthesis Activity. J. Phys. Chem. C 2017, 121, 20900–20904. [Google Scholar] [CrossRef]
- Kobayashi, V.; Kitano, M.; Kawamura, S.; Yokoyama, T.; Hosono, H. Kinetic evidence: The rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step. Catal. Sci. Technol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Li, J.; Kitano, M.; Ye, T.; Sasase, M.; Yokoyama, T.; Hosono, H. Chlorine-Tolerant Ruthenium Catalyst Derived Using the Unique Anion-Exchange Properties of 12CaO·7Al2O3 for Ammonia Synthesis. ChemCatChem 2017, 9, 3078–3083. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-Organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. Int. Ed. 2018, 57, 2648–2652. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Catalytic Reaction Assisted by Plasma or Electric Field. Chem. Rec. 2017, 17, 726–738. [Google Scholar] [CrossRef]
- Murakami, K.; Manabe, R.; Nakatsubo, H.; Yabe, T.; Ogo, S.; Sekine, Y. Elucidation of the role of electric field on low temperature ammonia synthesis using isotopes. Catal. Today 2018, 303, 271–275. [Google Scholar] [CrossRef]
- Manabe, R.; Nakatsubo, H.; Gondo, A.; Murakami, K.; Ogo, S.; Tsuneki, H.; Ikeda, M.; Ishikawa, A.; Nakai, H.; Sekine, Y. Electrocatalytic synthesis of ammonia by surface proton hopping. Chem. Sci. 2017, 8, 5434–5439. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Jing, B.; Lin, J.; Lin, B.; Zhao, Z.; Jiang, L. Effect of rare earth on the performance of Ru/MgAl-LDO catalysts for ammonia synthesis. J. Rare Earths 2018, 36, 135–141. [Google Scholar] [CrossRef]
- Ogura, Y.; Sato, K.; Miyahara, S.I.; Kawano, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Hosokawa, S.; Nagaoka, K. Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature. Chem. Sci. 2018, 9, 2230–2237. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Zhao, S.; Xiong, X.; Hu, B.; Song, C. Effect of Graphitic Carbon Nitride on the Electronic and Catalytic Properties of Ru Nanoparticles for Ammonia Synthesis. Catal. Lett. 2016, 146, 2324–2329. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 2003, 301, 76–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shima, T.; Hu, S.; Luo, G.; Kang, X.; Luo, Y.; Hou, Z. Dinitrogen cleavage and hydrogenation by a trinuclear titanium polyhydride complex. Science 2013, 340, 1549–1952. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Nishibayashi, Y. Developing more sustainable processes for ammonia synthesis. Coord. Chem. Rev. 2013, 257, 2551–2564. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishibayashi, Y.; Yoshizawa, K. Interplay between Theory and Experiment for Ammonia Synthesis Catalyzed by Transition Metal Complexes. Acc. Chem. Res. 2016, 49, 987–995. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Matsuo, Y.; Tanaka, H.; Ishii, K.; Yoshizawa, K.; Nishibayashi, Y. Catalytic transformation of dinitrogen into ammonia and hydrazine by iron-dinitrogen complexes bearing pincer ligand. Nat. Commun. 2016, 7, 12181. [Google Scholar] [CrossRef]
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-state electrochemical synthesis of ammonia: A review. J. Solid State Electrochem. 2011, 15, 1845–1860. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Khoenkhoen, N.; de Bruin, B.; Reek, J.N.H.; Dzik, W.I. Reactivity of Dinitrogen Bound to Mid- and Late-Transition-Metal Centers. Eur. J. Inorg. Chem. 2015, 2015, 567–598. [Google Scholar] [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium Nanocatalysts for Ammonia Synthesis: A Review. Chem. Eng. Commun. 2015, 202, 420–448. [Google Scholar] [CrossRef]
- Klinsrisuk, S.; Irvine, J.T.S. Electrocatalytic ammonia synthesis via a proton conducting oxide cell with BaCe0.5Zr0.3Y0.16Zn0.04O3-δ electrolyte membrane. Catal. Today 2017, 286, 41–50. [Google Scholar] [CrossRef]
- Kosaka, F.; Noda, N.; Nakamura, T.; Otomo, J. In situ formation of Ru nanoparticles on La1-xSrxTiO3-based mixed conducting electrodes and their application in electrochemical synthesis of ammonia using a proton-conducting solid electrolyte. J. Mater. Sci. 2017, 52, 2825–2835. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Bai, X.; Bai, M.; Ning, W. Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure. IEEE Trans. Plasma Sci. 2003, 31, 1285–1291. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.A. Bogaerts, Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma-catalysis: The known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Nakajima, J.; Sekiguchi, H. Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4446–4451. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. Plasma Chem. Plasma Process. 1989, 9, 13–24. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. II. Synthesis of ammonia in a microwave discharge under various conditions. Plasma Chem. Plasma Process. 1989, 9, 421–432. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Bai, M.; Bai, X.; Gao, H. Synthesis of ammonia using CH4/N2 plasmas based on micro-gap discharge under environmentally friendly condition. Plasma Chem. Plasma Process. 2008, 28, 405–414. [Google Scholar] [CrossRef]
- Peng, P.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; Zhou, N.; Schiappacasse, C.; Chen, D.; Zhang, Y.; Anderson, E.; Liu, Y.; et al. Ru-based multifunctional mesoporous catalyst for low-pressure and non-thermal plasma synthesis of ammonia. Int. J. Hydrogen Energy 2017, 42, 19056–19066. [Google Scholar] [CrossRef]
- van Helden, J.H.; Wagemans, W.; Yagci, G.; Zijlmans, R.A.B.; Schram, D.C.; Engeln, R.A.H.; Lombardi, G.; Stancu, D.; Ropcke, J. Detailed study of the plasma-activated catalytic generation of ammonia in N2-H2 plasmas. J. Appl. Phys. 2007, 101, 043305. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, T.; Mastumoto, K.; Ohkita, H.; Kakuta, N. Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge. Plasma Chem. Plasma Process. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Gomez-Ramirez, A.; Cotrino, J.; Lambert, R.M.; Conzalez-Elipe, A.R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 06501. [Google Scholar] [CrossRef]
- Gomez-Ramirez, A.; Montoro-Damas, A.; Cotrino, J.; Lambert, R.M.; Conzalez-Elipe, A.R. About the enhancement of chemical yield during the atmospheric plasma synthesis of ammonia in a ferroelectric packed bet reactor. Plasma Process. Polym. 2017, 14, e1600081. [Google Scholar] [CrossRef]
- Kim, H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem Plasma Process 2016, 36, 45–72. [Google Scholar] [CrossRef]
- Aihara, K.; Akiyama, M.; Deguchi, T.; Tanaka, M.; Hagiwara, R.; Iwamoto, M. Remarkable catalysis of a wool-like copper electrode for NH3 synthesis from N2 and H2 in non-thermal atmospheric plasma. Chem. Commun. 2016, 52, 13560–13563. [Google Scholar] [CrossRef]
- Iwamoto, M.; Akiyama, M.; Aihara, K.; Deguchi, T. Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. ACS Catal. 2017, 7, 6924–6929. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Liu, Y.; Ji, J.; Zhou, X.; Chen, D.; Yuan, W. Structure sensitivity of ammonia decomposition over Ni catalysts: A computational and experimental study. Fuel Process. Technol. 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Sato, K.; Abe, N.; Kawagoe, T.; Miyahara, S.; Honda, K.; Nagaoka, K. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int. J. Hydrogen Energy 2017, 42, 6610–6617. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak1, P.; Ambrozkiewicz1, W.; Sitko1, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Leltko, J. Ni-Supported Pd Nanoparticles with Ca Promoter: A New Catalyst for Low-Temperature Ammonia Cracking. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, F.; Shao, J.; Dai, Y.; Ding, J.; Tang, Z. Attapulgite clay supported Ni nanoparticles encapsulated by porous silica: Thermally stable catalysts for ammonia decomposition to COx free hydrogen. Int. J. Hydrogen Energy 2016, 41, 21157–21165. [Google Scholar] [CrossRef]
- Atsumi, R.; Noda, R.; Takagi, H.; Vecchione, L.; Carlo, A.D.; Del Prete, Z.; Kuramoto, K. Effects of Steam on Ni/Al2O3 Catalysts for Ammonia Decomposition. Ind. Eng. Chem. Res. 2014, 53, 17849–17853. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J.; Mckay, D. A comparison of the reactivity of lattice nitrogen in Co3Mo3N and Ni2Mo3N catalysts. J. Mol. Catal. A Chem. 2009, 305, 125–129. [Google Scholar] [CrossRef]
- Taylor, D.W.; Smith, P.J.; Dowdenc, D.A.; Kemball, C.; Whan, D.A. Ammonia synthesis and related reactions over iron-cobalt and iron-nickel alloy catalysts. Part I. Catalysts reduced at 853 K. Appl. Catal. 1982, 3, 161–176. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H. Novel class of ammonia synthesis catalysts. Chem. Commun. 2000, 1057–1058. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skulason, E. Computational Predictions of Catalytic Activity of Zincblende (110) Surfaces of Metal Nitrides for Electrochemical Ammonia Synthesis. J. Phys. Chem. C 2017, 121, 6141–6151. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhang, Y.; Zhang, Z.; Fan, H.J.; Rawat, R.S. Nitrogen-Plasma-Activated Hierarchical Nickel Nitride Nanocorals for Energy Applications. Small 2017, 13, 1604265. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, C.; Karim, W.; Ekinci, Y.; van Bokhoven, J.A.; VandeVondele, J. Hydrogen Adsorption on Nanosized Platinum and Dynamics of Spillover onto Alumina and Titania. J. Phys. Chem. C 2017, 121, 17862–17872. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Akande, A.J.; Idem, R.O.; Dalai, A.K. Synthesis, characterization and performance evaluation of Ni/Al2O3 catalysts for reforming of crude ethanol for hydrogen production. Appl. Catal. A Gen. 2005, 287, 159–175. [Google Scholar] [CrossRef]
- Ragupathi, C.; Bijaya, J.J.; Surendhar, P.; Kennedy, L.J. Comparative investigation of nickel aluminate (NiAl2O4) nano and microstructures for the structural, optical and catalytic properties. Polyhedron 2014, 72, 1–7. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Al-Noaimi, A.N.; Saber, T.M.H. Structure and catalytic activity of nickel oxide/alumina (NiO/Al2O3) prepared by impregnation or coprecipitation. Bull. Soc. Chim. France 1987, 930–934, CODEN BSCFAS. [Google Scholar]
- Jeevanandam, P.; Koltypin, Y.; Gedanken, A. Preparation of nanosized nickel aluminate spinel by a sonochemical method. Mater. Sci. Eng. B 2002, 90, 125–132. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Zhang, P.; Qi, R.; Huang, R.; Song, X.; Gao, L. Facile synthesis of hollow hierarchical Ni/γ-Al2O3 nanocomposites for methane dry reforming catalysis. RSC Adv. 2014, 4, 51184–51193. [Google Scholar] [CrossRef]
- Inoue, H.; Hatanaka, N.; Kidena, K.; Murata, S.; Nomura, M. Reforming of Methane with Carbon Dioxide over Nickel-loaded Zeolite Catalysts. J. Jpn. Petroleum Inst. 2002, 45, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Gong, Y.L.; Li, J.C.; Wang, Z.W. Influence of hydrothermal modification on the properties of Ni/Al2O3 catalyst. Appl. Surf. Sci. 2004, 239, 94–100. [Google Scholar] [CrossRef]
- Al-Ubaid, A.; Wolf, E.E. Steam reforming of methane on reduced non-stoichiometric nickel aluminate catalysts. Appl. Catal. 1988, 40, 73–85. [Google Scholar] [CrossRef]
- Salagre, P.; Fierro, J.L.G.; Medina, F.; Sueiras, J.E. Characterization of nickel species on several γ-alumina supported nickel samples. J. Mol. Catal. A Chem. 1996, 106, 125–134. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, X.; Zhang, L. Influence of Ni Loading on Catalytic Activity of NiO/γ-Al2O3 for Hydrogenation of Coal Pyrolysis. Asian J. Chem. 2013, 25, 5071–5075. [Google Scholar] [CrossRef]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.; Teunis-sen, H.T.; Chorkendorff, I. Ammonia synthesis with barium-promoted iron–cobalt alloys supported on carbon. J. Catal. 2003, 214, 327–335. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, M.; Horikoshi, M.; Hashimoto, R.; Shimano, K.; Sawaguchi, T.; Teduka, H.; Matsukata, M. Higher Activity of Ni/γ-Al2O3 over Fe/γ-Al2O3 and Ru/γ-Al2O3 for Catalytic Ammonia Synthesis in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. Catalysts 2020, 10, 590. https://doi.org/10.3390/catal10050590
Iwamoto M, Horikoshi M, Hashimoto R, Shimano K, Sawaguchi T, Teduka H, Matsukata M. Higher Activity of Ni/γ-Al2O3 over Fe/γ-Al2O3 and Ru/γ-Al2O3 for Catalytic Ammonia Synthesis in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. Catalysts. 2020; 10(5):590. https://doi.org/10.3390/catal10050590
Chicago/Turabian StyleIwamoto, Masakazu, Masataka Horikoshi, Ryu Hashimoto, Kaori Shimano, Tomiko Sawaguchi, Harunobu Teduka, and Masahiko Matsukata. 2020. "Higher Activity of Ni/γ-Al2O3 over Fe/γ-Al2O3 and Ru/γ-Al2O3 for Catalytic Ammonia Synthesis in Nonthermal Atmospheric-Pressure Plasma of N2 and H2" Catalysts 10, no. 5: 590. https://doi.org/10.3390/catal10050590
APA StyleIwamoto, M., Horikoshi, M., Hashimoto, R., Shimano, K., Sawaguchi, T., Teduka, H., & Matsukata, M. (2020). Higher Activity of Ni/γ-Al2O3 over Fe/γ-Al2O3 and Ru/γ-Al2O3 for Catalytic Ammonia Synthesis in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. Catalysts, 10(5), 590. https://doi.org/10.3390/catal10050590