Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils
Abstract
:1. Introduction
2. Results and Discussion
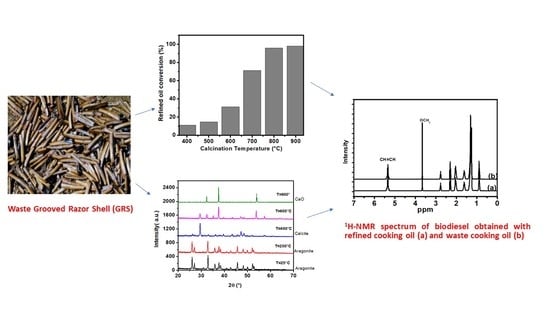
2.1. Materials Characterization
2.2. Oil Characterization
2.3. Catalytic Activity
2.3.1. Effect of Methanol/Oil Molar Ratio
2.3.2. Effect of Catalyst (GRS) Calcination Temperature on the Biodiesel Production
2.3.3. Catalyst Stability and Recycle
2.4. Characterization of Synthesized Biodiesel
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Catalyst Preparation
3.3. Characterization Techniques
3.4. Biodiesel Synthesis
4. Conclusions
- The optimal conditions for biodiesel production from waste cooking oil collected from Moroccan university restaurants and from refined oil were determined. The raw material that was used was characterized by good properties that were very close to the refined oil used for comparison.
- Calcium oxide obtained by calcination of waste GRS at 900 °C is a good performing catalyst for biodiesel production by the transesterification of waste oil.
- The biodiesel yield reached 94 wt% at a methanol/waste oil ratio of 15:1 and with 5 wt% of catalyst, without any additional cost due to water purification that is necessary when working in homogenous conditions.
- The 1H-NMR, FTIR and gas chromatography analyses of the final product confirmed that: (i) the reaction was complete, and that (ii) the biodiesel samples did not contain any trace of glycerol and (iii) met the required international standards.
- The catalyst, in the form of CaO, was reused for up to five cycles, with a slight decrease in the biodiesel yield from 94–87%. After that, the catalyst was regenerated by washing with methanol in an ultrasounds bath and further calcination at 900 °C for 1 h, recovering an activity very close to that of the fresh catalyst.
- The obtained results can be considered as proof that marine and earth shells can be used as a source of catalysts for biodiesel production.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Aalst, M.K. The impacts of climate change on the risk of natural disasters. Disasters 2006, 30, 5–18. [Google Scholar] [CrossRef]
- Hendry, A.P.; Gotanda, K.M.; Svensson, E.I. Human influences on evolution, and the ecological and societal consequences. Philos. Trans. R. Soc. B Boil. Sci. 2017, 372, 20160028. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, D.; Tatsutani, M. Sustainable energy for developing countries. SAPI EN S. Surv. Perspect. Integr. Environ. Soc. 2009, 2, 1–16. [Google Scholar]
- Burke, M.J.; Stephens, J. Political power and renewable energy futures: A critical review. Energy Res. Soc. Sci. 2018, 35, 78–93. [Google Scholar] [CrossRef]
- Hassan, M.; Kalam, M. An Overview of Biofuel as a Renewable Energy Source: Development and Challenges. Procedia Eng. 2013, 56, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Shrirame, H.Y.; Panwar, N.L.; Bamniya, B.R. Bio Diesel from Castor Oil—A Green Energy Option. Low Carbon Econ. 2011, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ouanji, F.; Nachid, M.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Small scale biodiesel synthesis from waste frying oil and crude methanol in Morocco. Chin. J. Chem. Eng. 2016, 24, 1178–1185. [Google Scholar] [CrossRef]
- Moreira, K.S.; Júnior, L.S.M.; Monteiro, R.R.C.; De Oliveira, A.L.B.; Valle, C.P.D.; Freire, T.M.; Fechine, P.B.A.; De Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I. Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Bautista, F.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Conceição, M.M.; Candeia, R.A.; Dantas, H.J.; Soledade, L.; Fernandes, V.J.; Souza, A.G. Rheological Behavior of Castor Oil Biodiesel. Energy Fuels 2005, 19, 2185–2188. [Google Scholar] [CrossRef]
- Jabbari, H. Production of methyl ester biofuel from sunflower oil via transesterification reaction. Asian J. Nanosci. Mater. 2018, 1, 52–55. [Google Scholar]
- Narasimharao, K.; Lee, A.; Wilson, K. Catalysts in production of biodiesel: A review. J. Biobased Mater. Bioenergy 2007, 1, 19–30. [Google Scholar]
- Serio, M.D.; Cozzolino, M.; Giordano, M.; Tesser, R.; Patrono, P.; Santacesaria, E. From Homogeneous to Heterogeneous Catalysts in Biodiesel Production. Ind. Eng. Chem. Res. 2007, 46, 6379–6384. [Google Scholar] [CrossRef]
- Saifuddin, N.; Samiuddin, A.; Kumaran, P. A Review on Processing Technology for Biodiesel Production. Trends Appl. Sci. Res. 2015, 10, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Dall’Oglio, E.L.; Sousa, P.T.D., Jr.; Oliveira, P.T.D.J.; De Vasconcelos, L.G.; Parizotto, C.A.; Kuhnen, C.A. Use of heterogeneous catalysts in methylic biodiesel production induced by microwave irradiation. Química Nova 2014, 37, 411–417. [Google Scholar] [CrossRef]
- Sahu, G.; Gupta, N.K.; Kotha, A.; Saha, S.; Datta, S.; Chavan, P.; Kumari, N.; Dutta, P. A Review on Biodiesel Production through Heterogeneous Catalysis Route. ChemBioEng Rev. 2018, 5, 231–252. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, S.; Bin Abu Bakar, Z.; Razak, I.S.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Shehryar, M.; Islam, K.N. Processing and Characterization of Cockle Shell Calcium Carbonate (CaCO3) Bioceramic for Potential Application in Bone Tissue Engineering. J. Mater. Sci. Eng. 2013, 2, 132. [Google Scholar] [CrossRef]
- Khachani, M.; Hamidi, A.E.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca (OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Niju, S.; Begum, K.M.M.S.; Anantharaman, N. Enhancement of biodiesel synthesis over highly active CaO derived from natural white bivalve clam shell. Arab. J. Chem. 2016, 9, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Siriprom, W.; Kaewkhao, J.; Phachana, K.; Limsuwan, P. Crystal Structure and Morphology Dependence of the Phase of Mollusc Shell: A Case Study of XRD, SEM and ESR. J. Phys. Conf. Ser. 2011, 266, 012124. [Google Scholar] [CrossRef]
- Ghafar, S.L.M.A.; Hussein, M.Z.; Rukayadi, Y.; Zakaria, Z.A.B. Surface-functionalized cockle shell–based calcium carbonate aragonite polymorph as a drug nanocarrier. Nanotechnol. Sci. Appl. 2017, 10, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Pelmenschikov, A.G.; van Wolput, J.H.M.C.; Jhchent, P.J.; van Santed, R.A. (A,B,C) Triplet of Infrared OH Bands of Zeolitic H-Complexes. J. Phys. Chem. 1995, 99, 3612–3617. [Google Scholar] [CrossRef] [Green Version]
- Jurac, Z.; Zlatar, V. Optimization of raw material mixtures in the production of biodiesel from vegetable and used frying oils regarding quality requirements in terms of cold flow properties. Fuel Process. Technol. 2013, 106, 108–113. [Google Scholar] [CrossRef]
- Cukalovic, A.; Monbaliu, J.-C.M.; Eeckhout, Y.; Echim, C.; Verhé, R.; Heynderickx, G.; Stevens, C.V. Development, optimization and scale-up of biodiesel production from crude palm oil and effective use in developing countries. Biomass Bioenergy 2013, 56, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Kenar, J. Determination of the fatty acid profile by1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Conceição, M.M.; Candeia, R.A.; Silva, F.C.; Bezerra, A.F.; Fernandes, V.J., Jr.; Souza, A.G. Thermoanalytical characterization of castor oil biodiesel. Renew.Sustain. Energy Rev. 2007, 11, 964–975. [Google Scholar]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A.; Ali, D.M.H. Utilization of waste cockle shell (Anadara granosa) in biodiesel production from palm olein: Optimization using response surface methodology. Fuel 2011, 90, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Qasim, D.; Abdul-Aziz, Y.I.; Alismaeel, Z.T. Biodiesel from fresh and waste sunflower oil using calcium oxide catalyst synthesized from local limestone. Res. J. Chem. Environ. 2019, 23, 111–119. [Google Scholar]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of vegetable oils: A review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Savaliya, M.L.; Dhorajiya, B.D.; Dholakiya, B.Z. Current Trends in Separation and Purification of Fatty Acid Methyl Ester. Sep. Purif. Rev. 2014, 44, 28–40. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.; Jamil, F.; Al-Haj, L.; Myint, M.T.Z.; Mahmoud, E.; Ahmad, M.N.; Hasan, A.O.; Rafiq, S. Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnol. Rep. 2018, 20, e00284. [Google Scholar] [CrossRef]
- Sanchez-Cantu, M.; Perez-Diaz, L.M.; Rosas, I.P.; Cadena-Torres, E.; Juárez-Amador, L.; Rubio, E.; Rodríguez-Acosta, M.; Valente, J.S. Hydrated lime as an effective heterogeneous catalyst for the transesterification of castor oil and methanol. Fuel 2013, 110, 54–62. [Google Scholar] [CrossRef]
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, S.; Demshemino, I.; Yahaya, M.; Nwandike, I.; Okoro, L. A review on the spectroscopic analyses of biodiesel. Eur. Int. J. Sci. Technol. 2013, 2, 137–146. [Google Scholar]
- Tariq, M.; Shahzadi, S.; Ahmad, F.; Ahmad, M.; Zafar, M.; Khalid, N.; Khan, M.A. Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process. Technol. 2011, 92, 336–341. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; El Mahi, M.; Lotfi, E.M.; Kacimi, M.; Mahfoud, Z. Biodiesel synthesis from vegetable oil using eggshell waste as a heterogeneous catalyst. Biofuels 2019, 1–7. [Google Scholar] [CrossRef]













| Properties | Units | Refined Frying Oil | Used Frying Oil |
|---|---|---|---|
| Viscosity (40 °C) | cSt | 33.5 | 36.6 |
| Density (20 °C) | g/cm3 | 0.921 | 0.960 |
| Acidic value | mg KOH/g | 0.27 | 1.32 |
| Fatty acid type | Carbon chain | Composition (wt%) | |
| Myristic | C14:0 | - | 0.16 |
| Palmitic | C16:0 | 9.79 | 10.24 |
| Palmitoleic | C16:1 | traces | traces |
| Stearic | C18:0 | 3.66 | 3.85 |
| Oleic | C18:1 | 23.0 | 28.87 |
| Linoleic | C18:2 | 57.36 | 53.86 |
| Linolenic | C18:3 | 6.19 | 3.02 |
| Batches’ Characteristics | Refined Oil Biodiesel | Waste Oil Biodiesel | European Standards | |
|---|---|---|---|---|
| Maximum biodiesel yield | wt.% | 96.5 | 93.5 | - |
| Ester content | wt.% | 97 | 94.3 | ≥96.5 |
| Viscosity (40 °C) | mm2/s | 3.5 | 4.4 | 3.5–5.5 |
| Density (15 °C) | g/cm3 | 0.881 | 0.908 | 0.88–0.9 |
| Acid Value | mg KOH/g oil | 0.27 | 0.44 | 0.5 max |
| Water content | ppm | 348 | 456 | 500 max |
| Sulphur Ash content | mg/Kg | 5.6 | 7.8 | 10 max |
| Flash point | °C | 130.3 | 139 | 120 min |
| Cetane number | 49.5 | 52.2 | 51 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitlaalim, A.; Ouanji, F.; Benzaouak, A.; El Mahi, M.; Lotfi, E.M.; Kacimi, M.; Liotta, L.F. Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils. Catalysts 2020, 10, 703. https://doi.org/10.3390/catal10060703
Aitlaalim A, Ouanji F, Benzaouak A, El Mahi M, Lotfi EM, Kacimi M, Liotta LF. Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils. Catalysts. 2020; 10(6):703. https://doi.org/10.3390/catal10060703
Chicago/Turabian StyleAitlaalim, Abdellah, Fatiha Ouanji, Abdellah Benzaouak, Mohammed El Mahi, El Mostapha Lotfi, Mohamed Kacimi, and Leonarda Francesca Liotta. 2020. "Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils" Catalysts 10, no. 6: 703. https://doi.org/10.3390/catal10060703
APA StyleAitlaalim, A., Ouanji, F., Benzaouak, A., El Mahi, M., Lotfi, E. M., Kacimi, M., & Liotta, L. F. (2020). Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils. Catalysts, 10(6), 703. https://doi.org/10.3390/catal10060703