A Pd/MnO2 Electrocatalyst for Nitrogen Reduction to Ammonia under Ambient Conditions
Abstract
:1. Introduction
2. Results and Discussion
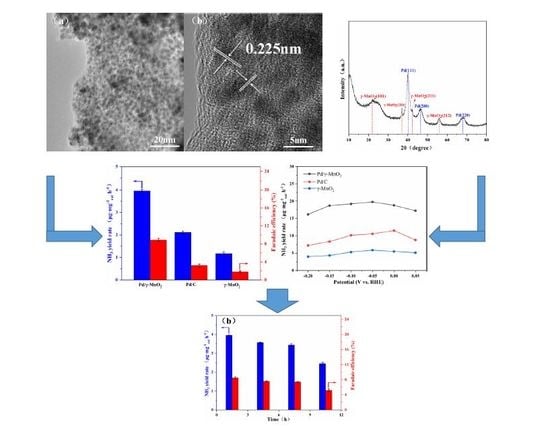
2.1. Characterization of Catalyst
2.2. Electroreduction of N2 to NH3 on Pd/γ-MnO2 Catalyst
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. α-MnO2
3.2.2. β-MnO2
3.2.3. γ-MnO2
3.2.4. Pd/γ-MnO2
3.3. Preparations of the Working Electrodes
3.4. Ammonia Quantification
3.5. Calculation of the NH3 Yield Rate the Faradaic Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klerke, A.; Christensen, C.H.; Norskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. A 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Furuya, N.; Yoshiba, H. Electroreduction of nitrogen to ammonia on gas-diffusion electrodes modified by Fe-phthalocyanine. J. Electroanal. Chem. 1989, 263, 171–174. [Google Scholar] [CrossRef]
- Furuya, N.; Yoshiba, H. Electroreduction of nitrogen to ammonia on gas-diffusion electrodes loaded with inorganic catalyst. J. Electroanal. Chem. 1990, 291, 269–272. [Google Scholar] [CrossRef]
- Kordali, V.; Kyriacou, G.; Lambrou, C. Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem. Commun. 2000, 48, 1673–1674. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef]
- Service, R.F. New recipe produces ammonia from air, water, and sunlight. Science 2014, 345, 610. [Google Scholar] [CrossRef] [PubMed]
- Van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Zhou, F.; Chen, K.; Kotzur, C.; Xiao, C.; Bourgeois, L.; Zhang, X.; MacFarlane, D.R. Nanostructured photoelectrochemical solar cell for nitrogen reduction using plasmon-enhanced black silicon. Nat. Commun. 2016, 7, 11335. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-driven dinitrogen reduction catalyzed by a CdS: Nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.N.; Greenlee, L.F.; Herring, A.M.; Ayers, K.E. Electrochemical synthesis of ammonia: A low pressure, low temperature approach. Electrochem. Soc. Interface 2015, 24, 51–57. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E.; Vourros, A.; Stoukides, M. Progress in the electrochemical synthesis of ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Soloveichik, G.L. Liquid fuel cells. Beilstein J. Nanotechnol. 2014, 5, 1399–1418. [Google Scholar] [CrossRef]
- Sheets, B.L.; Botte, G.G. Electrochemical nitrogen reduction to ammonia under mild conditions enabled by a polymer gel electrolyte. Chem. Commun. 2018, 54, 4250–4253. [Google Scholar] [CrossRef]
- Bao, D.; Zhang, Q.; Meng, F.L.; Zhong, H.X.; Shi, M.M.; Zhang, Y.; Yan, J.M.; Jiang, Q.; Zhang, X.B. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv. Mater. 2017, 29, 1–5. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Yu, H.; Xu, Y.; Xue, H.; Li, X.; Wang, H.; Wang, L. Ambient electrochemical synthesis of ammonia from nitrogen and water catalyzed by flower-like gold microstructures. ChemSusChem 2018, 11, 3480–3485. [Google Scholar] [CrossRef]
- Tsuneto, A.; Kudo, A.; ChemInform, T.S.J. Lithium-Mediated Electrochemical reduction of high Pressure N2 to NH3. J. Electroanal. Chem. 1994, 367, 183–188. [Google Scholar] [CrossRef]
- Nash, J.; Yang, X.; Anibal, J.; Wang, J.; Yan, Y.; Xu, B. Electrochemical nitrogen reduction reaction on noble metal catalysts in proton and hydroxide exchange membrane electrolyzers. J. Electroanal. Chem. 2017, 164, F1712–F1716. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, L.; Hu, L.; Chen, G.; Xin, H.; Feng, X. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 2018, 8, 1–25. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Electrochemical synthesis of ammonia directly from air and water using a Li+/H+/NH4+ mixed conducting electrolyte. RSC Adv. 2013, 3, 18016–18021. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Synthesis of ammonia directly from air and water at ambient temperature and pressure. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Chen, T.; Wang, Z. Electrochemical reduction of aqueous nitrogen (N2) at a low overpotential on (110)-oriented Mo nanofilm. J. Mater. Chem. A 2017, 5, 18967–18971. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, J.; Wei, Q.; Ma, Y.; Guo, H.; Liu, Q.; Wang, Y.; Cui, G.; Asiri, A.M.; Li, B.; et al. High-performance N2 to NH3 conversion electrocatalyzed by Mo2C nanorod. ACS Cent. Sci. 2019, 5, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, T.; Ma, Y.; Wei, Q.; Qiu, W.; Guo, H.; Shi, X.; Zhang, P.; Asiri, A.M.; Chen, L.; et al. Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower. Adv. Energy Mater. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.-Y.; Wang, H.; Ji, L.; Zhang, Y.; Chen, H.; Li, T.; Luo, Y.; Cui, G.; Sun, X. Boosting electrocatalytic N2 reduction by MnO2 with oxygen vacancies. Chem. Commun. 2019, 55, 4627–4630. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, F.; Zhang, L.; Wang, R.; Ji, L.; Liu, Q.; Luo, Y.; Guo, H.; Li, Y.; Gao, P.; et al. Electrocatalytic hydrogenation of N2 to NH3 by MnO: Experimental and theoretical investigations. Adv. Sci. 2019, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Bao, D.; Shi, M.M.; Wulan, B.R.; Yan, J.-M.; Jiang, Q. Amorphizing of Au nanoparticles by CeOx-RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions. Adv. Mater. 2017, 29, 1–6. [Google Scholar]
- Ma, J.L.; Bao, D.; Shi, M.M.; Yan, J.M.; Zhang, X.B. Reversible nitrogen fixation based on a rechargeable lithium-nitrogen battery for energy storage. Chem 2017, 2, 525–532. [Google Scholar] [CrossRef] [Green Version]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Norskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Cao, X.; Wu, S.; Zeng, X.; Ding, L.X.; Zhu, M.; Wang, H. Ammonia electrosynthesis with high selectivity under ambient conditions via a Li+ incorporation strategy. J. Am. Chem. Soc. 2017, 139, 9771–9774. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Kim, K.; Lee, N.; Yoo, C.Y.; Kim, J.N.; Yoon, H.C.; Han, J.I. Communication-electrochemical reduction of nitrogen to ammonia in 2-Propanol under ambient temperature and pressure. J. Electrochem. Soc. 2016, 163, F610–F612. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.M.; Chen, X.F.; Tian, Z.Q.; Zhang, X.Y.; Tsiakaras, P.; Shen, P.K. Electrocatalytic production of ammonia: Biomimetic electrode-electrolyte design for efficient electrocatalytic nitrogen fixation under ambient conditions. Appl. Catal. B 2020, 271, 1–10. [Google Scholar] [CrossRef]
- Ramaiyan, K.P.; Ozden, S.; Maurya, S.; Kelly, D.; Babu, S.K.; Benavidez, A.; Garzon, F.G.; Kim, Y.S.; Kreller, C.R.; Mukundan, R. Molybdenum carbide electrocatalysts for electrochemical synthesis of ammonia from nitrogen: Activity and stability. J. Electrochem. Soc. 2020, 167, 1–10. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdottir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jonsson, H.; Norskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef] [Green Version]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Norskov, J.K. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Vojvodic, A.; Norskov, J.K. New design paradigm for heterogeneous catalysts. Natl. Sci. Rev. 2015, 2, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Wickman, B. Depth probing of the hydride formation process in thin Pd films by combined electrochemistry and fiber optics-based in situ UV/vis spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 18953–18960. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Linke, U.; Wandlowski, T. Preparation and electrochemical characterization of palladiumsingle crystal electrodes in 0.1 M H2SO4 and HClO4 part I. low-index phases. Electrochim. Acta 2007, 52, 5733–5748. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Cai, F.; Wang, D.; Hu, Y.; Jiang, B.; Cai, W.B.; Chen, X.; Si, R.; Yang, F.; et al. Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles. Nano Res. 2017, 10, 2181–2191. [Google Scholar] [CrossRef]

















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Mu, Y.; Wang, Y. A Pd/MnO2 Electrocatalyst for Nitrogen Reduction to Ammonia under Ambient Conditions. Catalysts 2020, 10, 802. https://doi.org/10.3390/catal10070802
Sun C, Mu Y, Wang Y. A Pd/MnO2 Electrocatalyst for Nitrogen Reduction to Ammonia under Ambient Conditions. Catalysts. 2020; 10(7):802. https://doi.org/10.3390/catal10070802
Chicago/Turabian StyleSun, Chang, Yingxin Mu, and Yuxin Wang. 2020. "A Pd/MnO2 Electrocatalyst for Nitrogen Reduction to Ammonia under Ambient Conditions" Catalysts 10, no. 7: 802. https://doi.org/10.3390/catal10070802
APA StyleSun, C., Mu, Y., & Wang, Y. (2020). A Pd/MnO2 Electrocatalyst for Nitrogen Reduction to Ammonia under Ambient Conditions. Catalysts, 10(7), 802. https://doi.org/10.3390/catal10070802