Renewable Butene Production through Dehydration Reactions over Nano-HZSM-5/γ-Al2O3 Hybrid Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catatalytic Performance for the Dehydration of n-Butanol
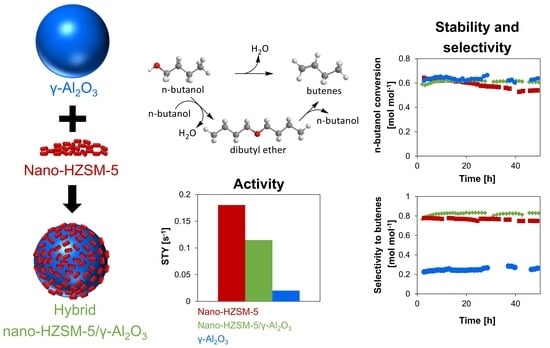
2.2.1. Activity Analysis
2.2.2. Selectivity Analysis
2.2.3. Catalyst Stability
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.Q.; Tan, T.W. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, Z.H. Recent progress on industrial fermentative production of acetone-butanol-ethanol by Clostridium acetobutylicum in China. Appl. Microbiol. Biotechnol. 2009, 83, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H. Bio-based economies in Asia: Economic analysis of development of bio-based industry in China, India, Japan, Korea, Malaysia and Taiwan. Int. J. Hydrog. Energy 2016, 41, 4333–4346. [Google Scholar] [CrossRef]
- Waldron, K.W. Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Weber, C.; Farwick, A.; Benisch, F.; Brat, D.; Dietz, H.; Subtil, T.; Boles, E. Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl. Microbiol. Biotechnol. 2010, 87, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Enguídanos, M.; Soria, A.; Kavalov, B.; Jensen, P. Techno-economic analysis of bio-alcohol production in the eu: A short summary for decision-makers. Eur. Comm. Rep. EUR 2002, 20280. [Google Scholar]
- Hergueta, C.; Tsolakis, A.; Herreros, J.M.; Bogarra, M.; Price, E.; Simmance, K.; York, A.P.E.; Thompsett, D. Impact of bio-alcohol fuels combustion on particulate matter morphology from efficient gasoline direct injection engines. Appl. Energy 2018, 230, 794–802. [Google Scholar] [CrossRef]
- He, Z.; Yang, M.; Wang, X.; Zhao, Z.; Duan, A. Effect of the transition metal oxide supports on hydrogen production from bio-ethanol reforming. Catal. Today 2012, 194, 2–8. [Google Scholar] [CrossRef]
- Shylesh, S.; Kim, D.; Ho, C.R.; Johnson, G.R.; Wu, J.; Bell, A.T. Non-Oxidative Dehydrogenation Pathways for the Conversion of C2–C4 Alcohols to Carbonyl Compounds. ChemSusChem 2015, 8, 3959–3962. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H. Propene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, BW, Germany, 2013. [Google Scholar]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, BW, Germany, 2000. [Google Scholar]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Dusselier, M.; Sels, B.F. Will Zeolite-Based Catalysis be as Relevant in Future Biorefineries as in Crude Oil Refineries? Angew. Chem. Int. Ed. 2014, 53, 8621–8626. [Google Scholar] [CrossRef]
- Kasztelan, S.; Payen, E.; Toulhoat, H.; Grimblot, J.; Bonnelle, J.P. Industrial MoO3-promoter oxide-γ-Al2O3 hydrotreating catalysts: Genesis and architecture description. Polyhedron 1986, 5, 157–167. [Google Scholar] [CrossRef]
- Wang, J.; Dong, L.; Hu, Y.; Zheng, G.; Hu, Z.; Chen, Y. Dispersion of NiO supported on γ-Al2O3 and TiO2/γ-Al2O3 supports. J. Solid State Chem. 2001, 157, 274–282. [Google Scholar] [CrossRef]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, Y.G.; Klocke, D.J.; Buchanan, J.S. Effects of high-level additions of ZSM-5 to a fluid catalytic cracking (FCC) RE-USY catalyst. Appl. Catal. A Gen. 1995, 131, 121–133. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Rui, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Li, X. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647–2652. [Google Scholar] [CrossRef]
- Odedairo, T.; Balasamy, R.J.; Al-Khattaf, S. Influence of mesoporous materials containing ZSM-5 on alkylation and cracking reactions. J. Mol. Catal. A Chem. 2011, 345, 21–36. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Müller, S.; Liu, Y.; Vishnuvarthan, M.; Sun, X.; van Veen, A.C.; Haller, G.L.; Sanchez-Sanchez, M.; Lercher, J.A. Coke formation and deactivation pathways on H-ZSM-5 in the conversion of methanol to olefins. J. Catal. 2015, 325, 48–59. [Google Scholar] [CrossRef]
- Sheng, Q.; Ling, K.; Li, Z.; Zhao, L. Effect of steam treatment on catalytic performance of HZSM-5 catalyst for ethanol dehydration to ethylene. Fuel Process. Technol. 2013, 110, 73–78. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Strategies to Enhance the Catalytic Performance of ZSM-5 Zeolite in Hydrocarbon Cracking: A Review. Catalysts 2017, 7, 367. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Euzen, P.; Raybaud, P.; Krokidis, X.; Toulhoat, H.; Le Loarer, J.-L.; Jolivet, J.-P.; Froidefond, C. Alumina. In Handbook of Porous Solids; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, BW, Germany, 2008. [Google Scholar]
- Coulier, L.; Kishan, G.; van Veen, J.A.R.; Niemantsverdriet, J.W. Influence of Support-Interaction on the Sulfidation Behavior and Hydrodesulfurization Activity of Al2O3-Supported W, CoW, and NiW Model Catalysts. J. Phys. Chem. B 2002, 106, 5897–5906. [Google Scholar] [CrossRef]
- López Cordero, R.; López Agudo, A. Effect of water extraction on the surface properties of Mo/Al2O3 and NiMo/Al2O3 hydrotreating catalysts. Appl. Catal. A Gen. 2000, 202, 23–35. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Javier Garcia-Garcia, F.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Mahzar, Z.A.S.; Radiman, S.; Hamid, A.M.A.; Khalilabad, S.R. Facile route for preparation of highly crystalline γ-Al2O3 nanopowder. Mater. Lett. 2012, 72, 32–35. [Google Scholar] [CrossRef]
- Gunst, D.; Alexopoulos, K.; Van Der Borght, K.; John, M.; Galvita, V.; Reyniers, M.-F.; Verberckmoes, A. Study of butanol conversion to butenes over H-ZSM-5: Effect of chemical structure on activity, selectivity and reaction pathways. Appl. Catal. A Gen. 2017, 539, 1–12. [Google Scholar] [CrossRef]
- Gunst, D.; Sabbe, M.; Reyniers, M.-F.; Verberckmoes, A. Study of n-butanol conversion to butenes: Effect of Si/Al ratio on activity, selectivity and kinetics. Appl. Catal. A Gen. 2019, 582, 117101. [Google Scholar] [CrossRef]
- Fan, D.; Dai, D.J.; Wu, H.S. Ethylene Formation by Catalytic Dehydration of Ethanol with Industrial Considerations. Materials 2013, 6, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; DeWilde, J.F.; Bhan, A. Kinetics and Mechanism of Alcohol Dehydration on γ-Al2O3: Effects of Carbon Chain Length and Substitution. ACS Catal. 2015, 5, 602–612. [Google Scholar] [CrossRef]
- Roy, S.; Mpourmpakis, G.; Hong, D.Y.; Vlachos, D.G.; Bhan, A.; Gorte, R.J. Mechanistic Study of Alcohol Dehydration on γ-Al2O3. ACS Catal. 2012, 2, 1846–1853. [Google Scholar] [CrossRef]
- Chiang, H.; Bhan, A. Catalytic consequences of hydroxyl group location on the rate and mechanism of parallel dehydration reactions of ethanol over acidic zeolites. J. Catal. 2010, 271, 251–261. [Google Scholar] [CrossRef]
- Yakovleva, I.S.; Banzaraktsaeva, S.P.; Ovchinnikova, E.V.; Chumachenko, V.A.; Isupova, L.A. Catalytic Dehydration of Bioethanol to Ethylene. Catal. Ind. 2016, 8, 152–167. [Google Scholar] [CrossRef]
- Zhang, M.H.; Yu, Y.Z. Dehydration of Ethanol to Ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- de Reviere, A.; Gunst, D.; Sabbe, M.; Verberckmoes, A. Sustainable short-chain olefin production through simultaneous dehydration of mixtures of 1-butanol and ethanol over HZSM-5 and γ-Al2O3. J. Ind. Eng. Chem. 2020, 89, 257–272. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Γ-alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Shao, J.; Fu, T.; Ma, Q.; Ma, Z.; Zhang, C.; Li, Z. Controllable synthesis of nano-ZSM-5 catalysts with large amount and high strength of acid sites for conversion of methanol to hydrocarbons. Microporous Mesoporous Mater. 2019, 273, 122–132. [Google Scholar] [CrossRef]
- Qureshi, B.A.; Lan, X.; Arslan, M.T.; Wang, T. Highly Active and Selective Nano H-ZSM-5 Catalyst with Short Channels along b-axis for Glycerol Dehydration to Acrolein. Ind. Eng. Chem. Res. 2019, 58, 12611–12622. [Google Scholar] [CrossRef]
- Fu, T.; Chang, J.; Shao, J.; Li, Z. Fabrication of a nano-sized ZSM-5 zeolite with intercrystalline mesopores for conversion of methanol to gasoline. J. Energy Chem. 2017, 26, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Huangfu, J.J.; Mao, D.S.; Zhai, X.L.; Guo, Q.S. Remarkably enhanced stability of HZSM-5 zeolite co-modified with alkaline and phosphorous for the selective conversion of bio-ethanol to propylene. Appl. Catal. A Gen. 2016, 520, 99–104. [Google Scholar] [CrossRef]
- Takahashi, A.; Xia, W.; Nakamura, I.; Shimada, H.; Fujitani, T. Effects of added phosphorus on conversion of ethanol to propylene over ZSM-5 catalysts. Appl. Catal. A Gen. 2012, 423–424, 162–167. [Google Scholar] [CrossRef]
- Stein, A. Advances in Microporous and Mesoporous Solids—Highlights of Recent Progress. Adv. Mater. 2003, 15, 763–775. [Google Scholar] [CrossRef]
- Karlsson, A.; Stöcker, M.; Schäfer, K. In situ Synthesis of Micro- and Mesoporous Al-MFI/MCM-41 like Phases with High Hydrothermal Stability. In Studies in Surface Science and Catalysis; Sayari, A., Jaroniec, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 129, pp. 99–106. [Google Scholar]
- Habib, S.; Launay, F.; Laforge, S.; Comparot, J.-D.; Faust, A.-C.; Millot, Y.; Onfroy, T.; Montouillout, V.; Magnoux, P.; Paillaud, J.-L.; et al. High catalytic cracking activity of Al-MCM-41 type materials prepared from ZSM-5 zeolite crystals and fumed silica. Appl. Catal. A Gen. 2008, 344, 61–69. [Google Scholar] [CrossRef]
- Chen, G.W.; Li, S.L.; Jiao, F.J.; Yuan, Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal. Today 2007, 125, 111–119. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.-J.; Yan, X.; Zhou, Y.-J.; Lin, Z.-N.; Mi, S.; Cheng, K.-K.; Zhang, J.-A. Efficient Catalytic Dehydration of High-Concentration 1-butanol with Zn-Mn-Co modified γ-Al2O3 in Jet Fuel Production. Catalysts 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Jiao, Q.; Fang, Z.; Li, T.; Feng, C.; Li, H.; Zhao, Y. Light olefin production from catalytic pyrolysis of waste tires using nano-HZSM-5/γ-Al2O3 catalysts. J. Anal. Appl. Pyrolysis 2018, 129, 66–71. [Google Scholar] [CrossRef]
- Qin, Z.; Pinard, L.; Benghalem, M.A.; Daou, T.J.; Melinte, G.; Ersen, O.; Asahina, S.; Gilson, J.-P.; Valtchev, V. Preparation of Single-Crystal “House-of-Cards”-like ZSM-5 and Their Performance in Ethanol-to-Hydrocarbon Conversion. Chem. Mater. 2019, 31, 4639–4648. [Google Scholar] [CrossRef]
- van Steen, E.; Claeys, I.M.; Callanan, L.H. (Eds.) Recent Advances in the Science and Technology of Zeolites and Related materials. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 154. [Google Scholar]
- Huang, S.; Zhao, Z.; Chen, X.; Li, F. Alkali extraction of valuable metals from spent Mo–Ni/Al2O3 catalyst. Int. J. Refract. Met. Hard Mater. 2014, 46, 109–116. [Google Scholar] [CrossRef]
- Eder, F.; Stockenhuber, M.; Lercher, J.A. Brønsted Acid Site and Pore Controlled Siting of Alkane Sorption in Acidic Molecular Sieves. J. Phys. Chem. B 1997, 101, 5414–5419. [Google Scholar] [CrossRef]
- Wang, Z.; O’Dell, L.A.; Zeng, X.; Liu, C.; Zhao, S.; Zhang, W.; Gaborieau, M.; Jiang, Y.; Huang, J. Insight into Three-Coordinate Aluminum Species on Ethanol-to-Olefin Conversion over ZSM-5 Zeolites. Angew. Chem. Int. Ed. 2019, 58, 18061–18068. [Google Scholar] [CrossRef]
- Larmier, K.; Chizallet, C.; Cadran, N.; Maury, S.; Abboud, J.; Lamic-Humblot, A.-F.; Marceau, E.; Lauron-Pernot, H. Mechanistic Investigation of Isopropanol Conversion on Alumina Catalysts: Location of Active Sites for Alkene/Ether Production. ACS Catal. 2015, 5, 4423–4437. [Google Scholar] [CrossRef]
- Castaño, P.; Ruiz-Martínez, J.; Epelde, E.; Gayubo, A.G.; Weckhuysen, B.M. Spatial Distribution of Zeolite ZSM-5 within Catalyst Bodies Affects Selectivity and Stability of Methanol-to-Hydrocarbons Conversion. ChemCatChem 2013, 5, 2827–2831. [Google Scholar] [CrossRef]
- Song, W.; Justice, R.E.; Jones, C.A.; Grassian, V.H.; Larsen, S.C. Synthesis, Characterization, and Adsorption Properties of Nanocrystalline ZSM-5. Langmuir 2004, 20, 8301–8306. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Van der Borght, K.; Galvita, V.V.; Marin, G.B. Ethanol to higher hydrocarbons over Ni, Ga, Fe-modified ZSM-5: Effect of metal content. Appl. Catal. A Gen. 2015, 492, 117–126. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature with Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Berger, R.J.; Stitt, E.H.; Marin, G.B.; Kapteijn, F.; Moulijn, J.A. Eurokin-Chemical Reaction Kinetics in Practice. Cattech 2001, 5, 30–60. [Google Scholar] [CrossRef]









| Material | Porosity | Acid Type | Acid Strength | Crystallinity |
|---|---|---|---|---|
| HZSM-5 | microporous | mostly Brønsted | strong | high |
| γ-Al2O3 | mesoporous | Lewis | medium | low to semi |
| Catalyst | SBET (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) a | Vmicro (cm3 g−1) | Vt (cm3 g−1) |
|---|---|---|---|---|---|
| c-HZSM-5 | 410 | 230 | 180 | 0.11 | 0.24 |
| n-HZSM-5 | 370 | 280 | 90 | 0.11 | 0.16 |
| PM-50/50 | 310 | 130 | 180 | 0.063 | 0.45 |
| Hybrid-50/50 | 303 | 172 | 131 | 0.084 | 0.26 |
| γ-Al2O3 | 195 | 2 | 193 | 0.00 | 0.49 |
| Catalyst | Amount of Acid Sites (µmol g−1) | Ed (kJ mol−1) a | |||
|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | ||
| c-HZSM-5 | 156 | 68 | 199 | 422 | 130 |
| n-HZSM-5 | 118 | 83 | 193 | 394 | 150 |
| PM-50/50 | 154 | 70 | 152 | 376 | 115 |
| Hybrid-50/50 | 129 | 94 | 50 | 273 | 128 |
| γ-Al2O3 | 137 | 123 | 190 | 450 | 92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Reviere, A.; Vandevyvere, T.; Sabbe, M.K.; Verberckmoes, A. Renewable Butene Production through Dehydration Reactions over Nano-HZSM-5/γ-Al2O3 Hybrid Catalysts. Catalysts 2020, 10, 879. https://doi.org/10.3390/catal10080879
de Reviere A, Vandevyvere T, Sabbe MK, Verberckmoes A. Renewable Butene Production through Dehydration Reactions over Nano-HZSM-5/γ-Al2O3 Hybrid Catalysts. Catalysts. 2020; 10(8):879. https://doi.org/10.3390/catal10080879
Chicago/Turabian Stylede Reviere, Arno, Tom Vandevyvere, Maarten K. Sabbe, and An Verberckmoes. 2020. "Renewable Butene Production through Dehydration Reactions over Nano-HZSM-5/γ-Al2O3 Hybrid Catalysts" Catalysts 10, no. 8: 879. https://doi.org/10.3390/catal10080879
APA Stylede Reviere, A., Vandevyvere, T., Sabbe, M. K., & Verberckmoes, A. (2020). Renewable Butene Production through Dehydration Reactions over Nano-HZSM-5/γ-Al2O3 Hybrid Catalysts. Catalysts, 10(8), 879. https://doi.org/10.3390/catal10080879