Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production
Abstract
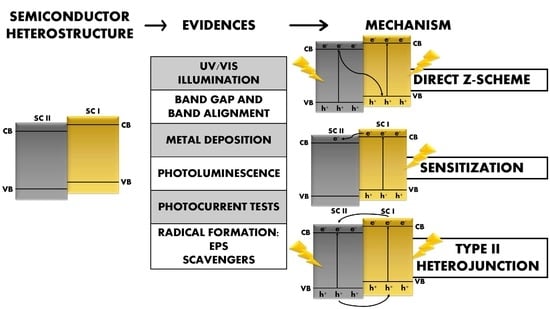
:1. Introduction
2. Charge Transfer Mechanisms: Concept and Relevance in Photocatalysis
3. Charge Transfer Mechanisms: Supporting Experimental Evidence
3.1. Sensitization
3.2. Direct Z-Scheme
3.3. Type II Heterojunction
4. Conclusions and Further Perspectives
Funding
Conflicts of Interest
References
- Schneider, J.; Kandiel, T.A.; Bahnemann, D. Solar photocatalytic hydrogen production: Current status and future challenges. In Nanostructure Science and Technology; Viswanathan, B., Subramanian, V. (Ravi), Lee, J.S., Eds.; Springer: New York, NY, USA, 2014; Volume 174, pp. 41–74. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F.; Sordello, F.; Minella, M.; Minero, C.; Maurino, V. The role of surface texture on the photocatalytic H2 production on TiO2. Catalysts 2019, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Michaelides, E.E. (Stathis) (Ed.) Energy Storage BT—Alternative Energy Sources; Springer: Berlin/Heidelberg, Germany, 2012; pp. 343–381. ISBN 978-3-642-20951-2. [Google Scholar]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.W.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2019, 120, 919–985. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, G.; Lim, H.; Zhu, C.; You, H.; Kim, Y.T. Effects of transition metal doping in Pt/M-TiO2 (M = V, Cr, and Nb) on oxygen reduction reaction activity. J. Power Sources 2016, 320, 188–195. [Google Scholar] [CrossRef]
- Hernandez, J.V.; Coste, S.; Murillo, A.G.; Romo, F.D.J.C.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloy. Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Ray, C.; Pal, T. Recent advances of metal–metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Wang, L.; Xu, X.; Gan, L.; Si, Z.; Li, J.; Zhang, Q.; Liu, Y.; Zhao, Y.; et al. Atomic palladium on graphitic carbon nitride as a hydrogen evolution catalyst under visible light irradiation. Commun. Chem. 2019, 2, 2–9. [Google Scholar] [CrossRef]
- Ribao, P.; Esteves, M.A.; Fernandes, V.R.; Rivero, M.J.; Rangel, C.; Ortiz, I. Challenges arising from the use of TiO2/rGO/Pt photocatalysts to produce hydrogen from crude glycerol compared to synthetic glycerol. Int. J. Hydrog. Energy 2019, 44, 28494–28506. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.; Hamilton, J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Chowdhury, P.; Malekshoar, G.; Ray, A.K.; Chowdhury, P. Dye-sensitized photocatalytic water splitting and sacrificial hydrogen generation: Current status and future prospects. Inorganics 2017, 5, 34. [Google Scholar] [CrossRef]
- Wu, H.L.; Li, X.B.; Tung, C.H.; Wu, L.Z. Recent advances in sensitized photocathodes: From molecular dyes to semiconducting quantum dots. Adv. Sci. 2018, 5, 1700684. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Qu, D.; An, L.; Gao, X.; Wen, Y.; Wang, X.; Sun, Z. Deliberate construction of direct Z-scheme photocatalysts through photodeposition. J. Mater. Chem. A 2019, 7, 18348–18356. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Qu, P.; Xu, Q.; Zheng, J.; Jia, S.; Chen, J.; Zhu, Z. A 2D/1D TiO2 nanosheet/CdS nanorods heterostructure with enhanced photocatalytic water splitting performance for H2 evolution. Int. J. Hydrog. Energy 2018, 43, 7388–7396. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, H.J.; Li, C.F.; Liu, J.; Dong, W.; Zhao, H.; Wang, C.; Liu, Y.; Hu, Z.Y.; Chen, L.; et al. Type II heterojunction in hierarchically porous zinc oxide/graphitic carbon nitride microspheres promoting photocatalytic activity. J. Colloid Interface Sci. 2019, 538, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, S.; Huebra, M.; Han, C.; Campo, P.; Nadagouda, M.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Magnetically recoverable TiO2-WO3 photocatalyst to oxidize bisphenol A from model wastewater under simulated solar light. Environ. Sci. Pollut. Res. 2016, 24, 12589–12598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanraju, K. Photoelectrochemical, photocatalytic and photochromic performance of rGO-TiO2-WO3 composites. Mater. Chem. Phys. 2019, 224, 217–228. [Google Scholar] [CrossRef]
- Si, Y.H.; Chen, W.M.; Shang, S.K.; Xia, Y.; Zeng, X.-R.; Zhou, J.; Li, Y.Y. g-C3N4/Pt/BiVO4 nanocomposites for highly efficient visible-light photocatalytic removal of contaminants and hydrogen generation. Nanotechnology 2020, 31, 125706. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Su, R.; Hu, B.; Wang, Z.; Jin, Y.; Wang, Y.; Besenbacher, F.; Dong, M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today 2019, 17, 159–182. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Kandi, D.; Martha, S.; Parida, K. Quantum dots as enhancer in photocatalytic hydrogen evolution: A review. Int. J. Hydrog. Energy 2017, 42, 9467–9481. [Google Scholar] [CrossRef]
- Rao, V.; Reddy, N.; Kumari, M.; Cheralathan, K.; Ravi, P.; Sathish, M.; Neppolian, B.; Reddy, K.R.; Shetti, N.P.; Prathap, P.; et al. Sustainable hydrogen production for the greener environment by quantum dots-based efficient photocatalysts: A review. J. Environ. Manag. 2019, 248, 109246. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrog. Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/electrochemical applications of metal Sulfide/TiO2 heterostructures. Adv. Energy Mater. 2019, 10, 1902355. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Borgarello, E.; Kiwi, J.; Graetzel, M.; Pelizzetti, E.; Visca, M. Visible light induced water cleavage in colloidal solutions of chromium-doped titanium dioxide particles. J. Am. Chem. Soc. 1982, 104, 2996–3002. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Y.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.; Wu, L.Z.; Tung, C.H.; Zhang, T. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Huang, H.; Li, H.; Han, X.; Liu, Y.; Kang, Z. Carbon quantum dot sensitized TiO2 nanotube arrays for photoelectrochemical hydrogen generation under visible light. Nanoscale 2013, 5, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Sargin, I.; Yanalak, G.; Arslan, G.; Patir, I.H. Green synthesized carbon quantum dots as TiO2 sensitizers for photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 21781–21789. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 evolution of metal-free g-C3N4 /SiC heterostructured photocatalysts. Appl. Surf. Sci. 2017, 391, 449–456. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- People, R.; Bean, J.C. Band alignments of coherently strained GexSi1−x/Si heterostructures on 〈001〉 GeySi1−y substrates. Appl. Phys. Lett. 1986, 48, 538–540. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P. Photosensitization of TiO2Nanostructures with CdS quantum dots: Particulate versus tubular support architectures. Adv. Funct. Mater. 2009, 19, 805–811. [Google Scholar] [CrossRef]
- Sun, W.T.; Yu, Y.; Pan, H.Y.; Gao, X.F.; Chen, Q.; Peng, L.M. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J. Am. Chem. Soc. 2008, 130, 1124–1125. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, D.; Dong, S.; Ren, N. Respective construction of Type-II and direct Z-scheme heterostructure by selectively depositing CdS on {001} and {101} facets of TiO2 nanosheet with CDots modification: A comprehensive comparison. J. Hazard. Mater. 2019, 366, 311–320. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Hu, J.; Liang, Y.H.; Cui, W. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core@shell Cu2O@g-C3N4 octahedra. Appl. Surf. Sci. 2015, 351, 1146–1154. [Google Scholar] [CrossRef]
- Xia, X.; Song, M.; Wang, H.; Zhang, X.; Sui, N.; Zhang, Q.; Colvin, V.L.; Yu, W.W. Latest progress in constructing solid-state Z scheme photocatalysts for water splitting. Nanoscale 2019, 11, 11071–11082. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. A Chem. 2002, 148, 71–77. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Idrees, F.; Dillert, R.; Bahnemann, D.W.; Butt, F.K.; Tahir, M. In-situ synthesis of Nb2O5/g-C3N4 heterostructures as highly efficient photocatalysts for molecular H2 evolution under solar illumination. Catalysts 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on metal sulphide-based z-scheme photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 structures doped with noble metals and/or graphene oxide to improve the photocatalytic degradation of dichloroacetic acid. Environ. Sci. Pollut. Res. 2016, 24, 12628–12637. [Google Scholar] [CrossRef]
- Capilli, G.; Costamagna, M.; Sordello, F.; Minero, C. Synthesis, characterization and photocatalytic performance of p-type carbon nitride. Appl. Catal. B: Environ. 2019, 242, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.T.; Carvalho, K.T.G.; Lopes, O.F.; Ribeiro, C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis: Enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation. Appl. Catal. B Environ. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously constructing heterojunction or direct Z-scheme photocatalysts by regulating electron flow direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Hu, X.; Lu, S.; Tian, J.; Wei, N.; Song, X.; Wang, X.; Cui, H. The selective deposition of MoS2 nanosheets onto (101) facets of TiO2 nanosheets with exposed (001) facets and their enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2019, 241, 329–337. [Google Scholar] [CrossRef]
- Isimjan, T.T.; Rasul, S.; Aloufi, M.N.; Khan, M.A.; Alhowaish, I.K.; Ahmed, T. Rational design of Pd-TiO2/g-C3N4 heterojunction with enhanced photocatalytic activity through interfacial charge transfer. Clean Energy 2019, 3, 59–68. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.T.; Qin, X.S.; Wu, S.J.; Zhao, H.; Yu, W.B.; Chen, L.H.; Liu, J.; Su, B.L.; Si-Jia, W.; et al. Light-assisted preparation of heterostructured g-C3N4/ZnO nanorods arrays for enhanced photocatalytic hydrogen performance. Catal. Today 2019, 1–5. [Google Scholar] [CrossRef]
- Liu, C.; Dong, S.; Chen, Y. Enhancement of visible-light-driven photocatalytic activity of carbon plane/g-C3N4/TiO2 nanocomposite by improving heterojunction contact. Chem. Eng. J. 2019, 371, 706–718. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, J.; Zhang, S.; Chen, G.; Meng, S.; Fan, Y.; Zheng, X.; Chen, S. What is the transfer mechanism of photoexcited charge carriers for g-C3N4/TiO2 heterojunction photocatalysts? Verification of the relative p–n junction theory. J. Phys. Chem. C 2020, 124, 8561–8575. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Wang, J.; Zhang, X.; Liu, S. Synthesis of CdTe/TiO2 nanoparticles and their photocatalytic activity. Mater. Res. Bull. 2013, 48, 4283–4286. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Jin, B.; Luo, J.; Xu, X.; Zhang, L.; Hong, Y. Heterojunctions in g-C3N4/B-TiO2 nanosheets with exposed {001} plane and enhanced visible-light photocatalytic activities. Int. J. Hydrog. Energy 2016, 41, 7292–7300. [Google Scholar] [CrossRef]
- Jo, W.K.; Selvam, N.C.S.; Adinaveen, T.; Vijaya, J.J. Synthesis of MoS2 nanosheet supported Z-scheme TiO2 /g-C3N4 photocatalysts for the enhanced photocatalytic degradation of organic water pollutants. RSC Adv. 2016, 6, 10487–10497. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, J.; Li, J.; Chen, J.; Xiang, Z.; Hu, W.; Li, M. Z-scheme TiO2/g-C3N4 composites with improved solar-driven photocatalytic performance deriving from remarkably efficient separation of photo-generated charge pairs. Mater. Res. Bull. 2016, 84, 65–70. [Google Scholar] [CrossRef]
- Fujihara, K.; Izumi, S.; Ohno, T.; Matsumura, M. Time-resolved photoluminescence of particulate TiO2 photocatalysts suspended in aqueous solutions. J. Photochem. Photobiol. A Chem. 2000, 132, 99–104. [Google Scholar] [CrossRef]
- LiQiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Fuer Tekn. Physik 1931, 12, 593–609. [Google Scholar]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hu, X.; Hu, J.; Wang, Z.; Yin, Y.; Sun, C.; Zhang, X. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Lin, X.; Xing, J.; Wang, W.; Shan, Z.; Xu, F.; Huang, F. Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: A strategy for the design of efficient combined photocatalysts. J. Phys. Chem. C 2007, 111, 18288–18293. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Sengele, A.; Keller, V.; Keller, N. Single-step synthesis of SnS2 nanosheet-decorated TiO2 anatase nanofibers as efficient photocatalysts for the degradation of gas-phase diethylsulfide. ACS Appl. Mater. Interfaces 2015, 7, 19324–19334. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chen, X.; Liu, W.; Tan, P.; Chen, H.; Wu, L.; Ma, C.; Xiong, X.; Pan, J. Photocorrosion inhibition and high-efficiency photoactivity of porous g-C3N4/Ag2CrO4 composites by simple microemulsion-assisted co-precipitation method. Appl. Catal. B Environ. 2017, 204, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Maheu, C.; Cardenas, L.; Puzenat, E.; Afanasiev, P.; Geantet, C. UPS and UV spectroscopies combined to position the energy levels of TiO2 anatase and rutile nanopowders. Phys. Chem. Chem. Phys. 2018, 20, 25629–25637. [Google Scholar] [CrossRef]
- Ong, W.J.; Putri, L.K.; Tan, L.L.; Li, N.; Ng, Y.H.; Wen, X.; Chai, S.P. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In situ irradiated X-ray photoelectron spectroscopy investigation on a direct Z-scheme TiO2/CdS composite film photocatalyst. Adv. Mater. 2018, 31, e1802981. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Vallejo, M.; Román, M.F.S.; Ortiz, I. Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafeiratos, S.; Jaén, J.J.D.; Fornasiero, P. Synthesis and photocatalytic application of visible-light active β -Fe2O3 /g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Jia, X.; Tahir, M.; Pan, L.; Huang, Z.F.; Zhang, X.; Wang, L.; Zou, J.J. Direct Z-scheme composite of CdS and oxygen-defected CdWO4: An efficient visible-light-driven photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2016, 198, 154–161. [Google Scholar] [CrossRef]
- Cui, H.; Li, B.; Zhang, Y.; Zheng, X.; Li, X.; Li, Z.; Xu, S. Constructing Z-scheme based CoWO4/CdS photocatalysts with enhanced dye degradation and H2 generation performance. Int. J. Hydrog. Energy 2018, 43, 18242–18252. [Google Scholar] [CrossRef]
- Obregón, S.; Ruíz-Gómez, M.; Rodríguez-González, V.; Vázquez, A.; Hernández-Uresti, D. A novel type-II Bi2W2O9/g-C3N4 heterojunction with enhanced photocatalytic performance under simulated solar irradiation. Mater. Sci. Semicond. Process. 2020, 113, 105056. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Meng, A.; Zhu, B.; Zhong, B.; Zhang, L.; Cheng, B. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 422, 518–527. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Han, C.; Likodimos, V.; Khan, J.A.; Nadagouda, M.N.; Andersen, J.; Falaras, P.; Rosales-Lombardi, P.; Dionysiou, D.D. UV–visible light-activated Ag-decorated, monodisperse TiO2 aggregates for treatment of the pharmaceutical oxytetracycline. Environ. Sci. Pollut. Res. 2013, 21, 11781–11793. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical intermediates in photoinduced reactions on TiO2 (An EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, Y.K.; Lim, S.K.; Kim, S.; In, S.I. Efficient visible light-induced H2 production by Au@CdS/TiO2 nanofibers: Synergistic effect of core–shell structured Au@CdS and densely packed TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 166, 423–431. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, B.; Pu, Y.; Liu, A.; Wang, J.; Ma, K.; Gao, F.; Gao, B.; Zou, W.; Dong, L. Interfacial coupling effects in g-C3N4/SrTiO3 nanocomposites with enhanced H2 evolution under visible light irradiation. Appl. Catal. B Environ. 2019, 247, 1–9. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A hierarchical Z-scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Raziq, F.; Li, C.; Humayun, M.; Qu, Y.; Zada, A.; Yu, H.; Jing, L. Synthesis of TiO2/g-C3N4 nanocomposites as efficient photocatalysts dependent on the enhanced photogenerated charge separation. Mater. Res. Bull. 2015, 70, 494–499. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, S.Z.; Qi, L.; Yu, J. Fabrication of NiS modified CdS nanorod p–n junction photocatalysts with enhanced visible-light photocatalytic H2-production activity. Phys. Chem. Chem. Phys. 2013, 15, 12088–12094. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xiong, H.; Hu, Y.; Han, J.; Rong, G.; Yin, Y. TiO2/NiO hybrid shells: P–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- Uddin, T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef]










| Reactive Species | Scavenger | References |
|---|---|---|
| •OH | Tert-butyl alcohol | [23,70,91,92] |
| Isopropanol | [21,52,67,68,83,93,94,95] | |
| Coumarin | [89,96,97] | |
| Terephthalic acid | [98] | |
| O2−• | Benzoquinone | [21,23,67,68,91,93,94,95] |
| TEMPO | [52,83] | |
| Potassium bromate | [70] | |
| Argon | [92] | |
| h+ | Formic acid | [91] |
| Methanol | [21,94] | |
| Sodium oxalate | [52,70] | |
| Ammonium oxalate | [67,68,93] | |
| Potassium iodide | [23,95] | |
| Triethanolamine | [92] | |
| H2O2 | Fe(II)-EDTA | [83] |
| Catalase | [95] |
| Mechanism | Catalyst | Objective | Sacrificial Agent | Light Source | Reference |
|---|---|---|---|---|---|
| Sensitization | TiO2/C | H2 production | Methanol | 500 W Xe lamp (λ > 450 nm) | [42] |
| TiO2/C | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (Visible region) | [43] | |
| TiO2/C | H2 production | TEOA | 300 W Xe lamp (λ > 420 nm) | [44] | |
| SiC/g-C3N4 | H2 production | TEOA | 300 W Xe lamp (λ > 420 nm) | [45] | |
| Pd/TiO2/g-C3N4 | H2 production | TEOA | 300 W Xe lamp (λ > 420 nm) | [74] | |
| TiO2/g-C3N4/C | Dye degradation | 500 W Xe lamp (λ > 400 nm) | [76] | ||
| TiO2/CdS | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (λ > 420 nm) | [101] | |
| TiO2/g-C3N4 | H2 production | TEOA | 300 W Xe lamp (λ > 420 nm) | [102] | |
| SrTiO3/g-C3N4 | H2 production | TEOA | 300 W Xe lamp (λ > 420 nm) | [103] | |
| TiO2/g-C3N4 | Dye degradation | 350 W Xe lamp (λ > 420 nm) | [104] | ||
| Direct Z-scheme | Fe2O3/g-C3N4 TiO2/CdS | H2 production | Na2S/Na2SO3 | 300 W Xe lamp | [21] |
| WO3/TiO2/Pt | H2 production | Methanol | 300 W Xe lamp | [25] | |
| g-C3N4/BiVO4 | H2 production | NaSO3 | 300 W Xe lamp (λ > 420 nm) | [27] | |
| TiO2/CdS | Pollutant removal | 150 W Xe lamp | [52] | ||
| g-C3N4/Nb2O5 | H2 production | TEOA/Methanol | 1000 W Xe lamp | [59] | |
| CoTiO3/g-C3N4/Pt | H2 production | Methanol | 300 W Xe lamp (λ > 420 nm) | [69] | |
| CdS/g-C3N4 | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (λ > 420 nm) | [72] | |
| Pt/TiO2/g-C3N4 | H2 production | TEOA | 350 W Xe lamp | [77] | |
| TiO2/g-C3N4 | Pollutant removal | UV radiation | [78] | ||
| TiO2/g-C3N4 | Pollutant removal | 500 W Xenon lamp | [67] | ||
| TiO2/g-C3N4 | Dye degradation | 500 W Xe lamp | [68] | ||
| TiO2/g-C3N4 | Water disinfection | 300 W Xe lamp | [83] | ||
| TiO2/CdS | CO2 reduction | 300 W Xe lamp | [89] | ||
| CdS/CdWO4 | H2 production | Lactic acid | 300 W Xe lamp (λ > 420 nm) | [93] | |
| CoWO4/CdS | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (λ > 420 nm) | [94] | |
| TiO2/g-C3N4 | Dye degradation | LED lamp 3 W (λ = 365 nm) | [96] | ||
| TiO2/CdS | H2 production | Methanol | 350 W Xe lamp | [97] | |
| ZnO/CdS | H2 production | Na2S/Na2SO3 | 350 W Xe lamp | [98] | |
| BiVO4/CdS | H2 production | Lactic acid | 300 W Xe lamp (λ > 420 nm) | [105] | |
| WO3/CdS | CO2 reduction | 300 W Xe lamp (λ > 420 nm) | [106] | ||
| αFe2O3/g-C3N4 | CO2 reduction | Visible light | [107] | ||
| Type II heterojunction | ZnO/g-C3N4 | Dye degradation | 300 W Xe lamp | [23] | |
| TiO2/CdS | Pollutant removal | 150 W Xe lamp | [52] | ||
| Cu2O/g-C3N4 | H2 production | TEOA | 300 W Xe lamp (λ > 400 nm) | [53] | |
| g-C3N4/Nb2O5 | Pollutant removal | 6x15 W visible light lamp | [70] | ||
| CdS/g-C3N4 | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (λ > 420 nm) | [72] | |
| TiO2/MoS2 | H2 production | TEOA | 300 W Xe lamp (λ>400 nm) | [73] | |
| ZnO/g-C3N4 | H2 production | Na2S/Na2SO3 | 300 W Xe lamp | [75] | |
| B-TiO2/g-C3N4 | H2 production | Methanol | 300 W Xe lamp (λ > 400 nm) | [66] | |
| βFe2O3/g-C3N4 | Dye degradation | 300 W Xe lamp (λ > 420 nm) | [92] | ||
| Bi2W2O9/g-C3N4 | Pollutant removal | 35 W Xe lamp | [95] | ||
| TiO2/g-C3N4 | H2 production | Methanol | 500 W Xe lamp | [108] | |
| NiS/CdS | H2 production | Na2S/Na2SO3 | 300 W Xe lamp (λ > 420 nm) | [109] | |
| TiO2/NiO | H2 production | Methanol | 300 W Xe lamp (λ > 400 nm) | [110] | |
| TiO2/NiO | H2 production | Methanol | 1.6 W Hg lamp (320 < λ < 400) | [111] |
| Supporting Information | |||
|---|---|---|---|
| Mechanism | Evidence | Characterization Technique | References |
| Common evidences to all charge transfer mechanisms | Reduction of charge recombination | Photoluminescence spectra | [23,27,42,43,44,45,49,52,53,58,63,64,65,66,67,68,69,70,71,74,93,95,96,101,102,103,104,106,108,109,110] |
| Band gap and band alignment | Diffuse reflectance | [23,27,42,43,44,45,49,52,53,58,63,64,65,66,67,68,69,70,71,74,93,95,96,101,102,103,104,106,108,109,110] | |
| XPS | |||
| Others (UPS, DFT) | |||
| Sensitization | Electron transfer | UV/Vis illumination | [42,43,44,45,74,76,101,102,103,104] |
| Direct Z-scheme | Radical generation | Radical trapping | [20,53,62,63,65,66,85,89,90,92,93,103] |
| EPR | [69,78,107] | ||
| Electron/hole active sites | Selective noble metal deposition | [21,72,93] | |
| Performance of different reactions | [69,105] | ||
| Photocorrosion | Catalyst reuse | [72,93,97] | |
| Transient photocurrent response | [52,94,97,98,106] | ||
| Type II heterojunction | Radical generation | Radical trapping | [23,27,52,70,92,95] |
| Electron/hole active sites | Selective noble metal deposition | [72] | |
| Performance of different reactions | [108] | ||
| Photocorrosion | Catalyst reuse | [53,75] | |
| Transient photocurrent response | [52,75,109] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Martín, S.; Rivero, M.J.; Ortiz, I. Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts 2020, 10, 901. https://doi.org/10.3390/catal10080901
San Martín S, Rivero MJ, Ortiz I. Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts. 2020; 10(8):901. https://doi.org/10.3390/catal10080901
Chicago/Turabian StyleSan Martín, Sergio, Maria J. Rivero, and Inmaculada Ortiz. 2020. "Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production" Catalysts 10, no. 8: 901. https://doi.org/10.3390/catal10080901
APA StyleSan Martín, S., Rivero, M. J., & Ortiz, I. (2020). Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts, 10(8), 901. https://doi.org/10.3390/catal10080901