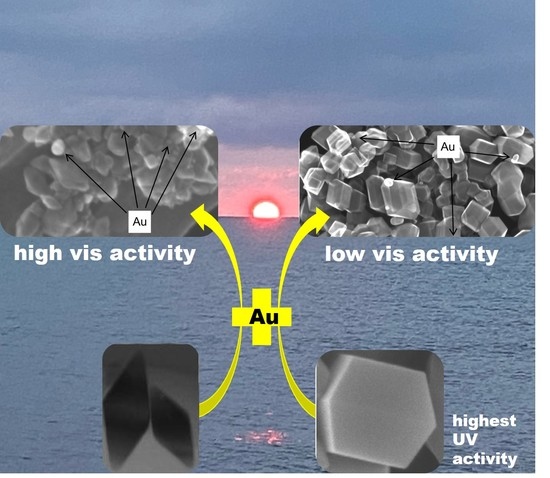
Morphology-Governed Performance of Plasmonic Photocatalysts
Abstract
:1. Introduction
2. Morphology Design of Plasmonic Photocatalysts
2.1. Faceted Semiconductors as Supports for Noble Metals
2.2. Other Particulate Plasmonic Photocatalysts with Advanced Morphology
2.3. One-Dimensional Plasmonic Photocatalysts
2.4. Two- and Three-Dimensional Plasmonic Photocatalysts
2.5. Other Plasmonic Photocatalysts
3. Summary
- (i)
- Better understanding of the destination photocatalytic reaction mechanism and synergistic effects between LSPR phenomenon and particle morphology is necessary—the usage of advanced characterization techniques to perform a detailed analysis of physicochemical properties of prepared materials coupled with providing theoretical simulations and calculations to deeply understand these synergistic interactions.
- (ii)
- The issue of the reaction selectivity by adjusting morphological properties of plasmonic photocatalysts (e.g., understanding the role of titania crystal facets configuration for the course of photocatalytic CO2 reduction) should be considered in more detail.
Funding
Conflicts of Interest
References
- Balcerski, W.; Ryu, S.Y.; Hoffmann, M.R. Visible-Light Photoactivity of Nitrogen-Doped TiO2: Photo-oxidation of HCO2H to CO2 and H2O. J. Phys. Chem. C 2007, 111, 15357–15362. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-light-induced water splitting based on Two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M. Heterogeneous photocatalysis: State of the art and present applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Wang, K.L.; Janczarek, M.; Wei, Z.S.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Katayama, S.; Nakamura, M.; Tsubota, T.; Ohno, T. Dependence of photocatalytic activity on aspect ratio of shape-controlled rutile titanium(IV) oxide nanorods. J. Phys. Chem. C 2011, 115, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Yasumoto, T.; Mahaney, O.O.P.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly active titania photocatalyst particles of controlled crystal phase, size, and polyhedral shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.S.; Kowalska, E.; Ohtani, B. Enhanced photocatalytic activity by particle morphology: Preparation, characterization, and photocatalytic activities of octahedral anatase titania particles. Chem. Lett. 2014, 43, 346–348. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Beranek, R.; Kisch, H. Surface-modified anodic TiO2 films for visible light photocurrent response. Electrochem. Commun. 2007, 9, 761–766. [Google Scholar] [CrossRef]
- Janus, M.; Tryba, B.; Inagaki, M.; Morawski, A.W. New preparation of a carbon-TiO2 photocatalyst by carbonization of n-hexane deposited on TiO2. Appl. Catal. B Environ. 2004, 52, 61–67. [Google Scholar] [CrossRef]
- Sclafani, A.; Mozzanega, M.N.; Pichat, P. Effect of silver deposits on the photocatalytic activity of titanium dioxide samples for the dehydrogenation or oxidation of 2-propanol. J. Photochem. Photobiol. A Chem. 1991, 59, 181–189. [Google Scholar] [CrossRef]
- Wang, K.L.; Endo-Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Boucle, J.; Ohtani, B.; Kowalska, E.; Herlin-Boime, N. Carbon/graphene-modified titania with enhanced photocatalytic activity under UV and vis irradiation. Materials 2019, 12, 4158. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, A.; Palmisano, L.; Venezia, A.M.; Augugliaro, V. Coupled semiconductor systems for photocatalysis. Preparation and characterization of polycrystalline mixed WO3/WS2 powders. J. Phys. Chem. B 1999, 103, 8236–8244. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Wang, K.L.; Kowalska, E. Synergistic effect of Cu2O and urea as modifiers of TiO2 for enhanced visible light activity. Catalysts 2018, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.-Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Morikawa, T.; Asahi, R.; Ohwaki, T.; Aoki, K.; Taga, Y. Band-gap narrowing of titanium dioxide by nitrogen doping. Jpn. J. Appl. Phys. 2001, 40, L561–L563. [Google Scholar] [CrossRef]
- Anpo, M. Use of visible light. Second-generation titanium oxide photocatalysts prepared by the application of an advanced metal ion-implantation method. Pure Appl. Chem. 2000, 72, 1787–1792. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cheng, H.M.; Zhang, L.; Hao, Y.Z.; Ma, J.M.; Xu, B.; Li, W.H. The preparation, characterization, photoelectrochemical and photocatalytic properties of lanthanide metal-ion-doped TiO2 nanoparticles. J. Mol. Catal. A Chem. 2000, 151, 205–216. [Google Scholar] [CrossRef]
- Hirano, K.; Suzuki, E.; Ishikawa, A.; Moroi, T.; Shiroishi, H.; Kaneko, M. Sensitization of TiO2 particles by dyes to achieve H2 evolution by visible light. J. Photochem. Photobiol. A 2000, 136, 157–161. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, G.; Tatsuma, T. Shape-controlled electrodeposition of gold nanostructures. J. Phys. Chem. B 2006, 110, 23478–23481. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonance enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.B.; Hung, W.H.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photochem. Photobiol. C 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Nishijima, Y.; Ueno, K.; Yokata, Y.; Murakoshi, K.; Misawa, H. Plasmon-assisted photocurrent generation from visible to near-infrared wavelength using a Au-nanorods/TiO2 electrode. J. Phys. Chem. Lett. 2010, 1, 2031–2036. [Google Scholar] [CrossRef]
- Bouhadoun, S.; Guillard, C.; Dapozze, F.; Singh, S.; Amans, D.; Boucle, J.; Herlin-Boime, N. One step synthesis of N-doped and Au-loaded TiO2 nanoparticles by laser pyrolysis: Application in photocatalysis. Appl. Catal. B Environ. 2015, 174, 367–375. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; Pavaskar, P.; Hsuan Hung, W.; Cronin, S.B. Plasmonic enhancement of photocatalytic decomposition of methyl orange under visible light. J. Catal. 2011, 277, 149–153. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.W.; Low, M.; Zhang, S.-Y.; Liu, Z.; Mlayah, A.; Han, M.-Y. Janus Au-TiO2 photocatalysts with strong localization of plasmonic near fields for efficient visible-light hydrogen generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Furube, A.; Hara, K.; Katoh, R.; Tachiya, M. Plasmon induced electron transfer at gold-TiO2 interface under femtosecond near-IR two photon excitation. Thin Solid Films 2009, 158, 861–864. [Google Scholar] [CrossRef]
- Du, L.C.; Furube, A.; Yamamoto, K.; Hara, K.; Katoh, R.; Tachiya, M. Plasmon-induced charge separation and recombination dynamics in gold-TiO2 nanoparticle systems: Dependence on TiO2 particle size. J. Phys. Chem. C 2009, 113, 6454–6462. [Google Scholar] [CrossRef]
- Caretti, I.; Keulemans, M.; Verbruggen, S.W.; Lenaerts, S.; Van Doorslaer, S. Light-induced processes in plasmonic gold/TiO2 photocatalysts studied by electron paramagnetic resonance. Top. Catal. 2015, 58, 776–782. [Google Scholar] [CrossRef]
- Priebe, J.B.; Radnik, J.; Lennox, A.J.J.; Pohl, M.M.; Karnahl, M.; Hollmann, D.; Grabow, K.; Bentrup, U.; Junge, H.; Beller, M.; et al. Solar hydrogen production by plasmonic Au-TiO2 catalysts: Impact of synthesis protocol and TiO2 phase on charge transfer efficiency and H2 evolution rates. ACS Catal. 2015, 5, 2137–2148. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodriguez-Lopez, J.L.; Remita, H. Surface modification of TiO2 with Au nanoclusters for efficient water treatment and hydrogen generation under visible light. J. Phys. Chem. C 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Fujiwara, Y.; Takahashi, Y.; Tatsuma, T. Plasmon-resonance-based generation of cathodic photocurrent at electrodeposited gold nanoparticles coated with TiO2 films. Chem. Phys. Chem. 2009, 10, 766–769. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.-Y.; Zhao, J.-C.; Zheng, Z.-F.; Gao, X.-P. Visible-light-driven oxidation of organic contaminants in air with gold nanoparticle catalysts on oxide supports. Angew. Chem. Int. Ed. 2008, 47, 5353–5356. [Google Scholar] [CrossRef]
- Wang, C.J.; Ranasingha, O.; Natesakhawat, S.; Ohodnicki, P.R.; Andio, M.; Lewis, J.P.; Matranga, C. Visible light plasmonic heating of Au-ZnO for the catalytic reduction of CO2. Nanoscale 2013, 5, 6968–6974. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E.S. Characterization and catalytic properties of nano-sized Au metal catalyst on titanium containing high mesoporous silica (Ti-HMS) synthesized by photo-sssisted deposition and impregnation methods. Int. J. Photoenergy 2011. [Google Scholar] [CrossRef]
- Trammell, S.A.; Nita, R.; Moore, M.; Zabetakis, D.; Chang, E.; Knight, D.A. Accelerating the initial rate of hydrolysis of methyl parathion with laser excitation using monolayer protected 10 nm Au nanoparticles capped with a Cu(bpy) catalyst. Chem. Commun. 2012, 48, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Li, J.T.; Meng, F.K.; Senty, T.R.; Suri, S.; Zhi, M.J.; Li, M.; Bristow, A.D.; Wu, N.Q. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Juarez, R.; Marino, T.; Molinari, R.; Garcia, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef]
- Son, M.S.; Im, J.E.; Wang, K.K.; Oh, S.L.; Kim, Y.R.; Yoo, K.H. Surface plasmon enhanced photoconductance and single electron effects in mesoporous titania nanofibers loaded with gold nanoparticles. Appl. Phys. Lett. 2010, 96, 023115. [Google Scholar] [CrossRef]
- Kominami, H.; Tanaka, A.; Hashimoto, K. Gold nanoparticles supported on cerium(IV) oxide powder for mineralization of organic acids in aqueous suspensions under irradiation of visible light of λ = 530 nm. Appl. Catal. A Gen. 2011, 397, 121–126. [Google Scholar] [CrossRef]
- Ilie, M.; Cojocaru, B.; Parvulescu, V.I.; Garcia, H. Improving TiO2 activity in photo-production of hydrogen from sugar industry wastewaters. Int. J. Hydrogen Energy 2011, 36, 15509–15518. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Wei, Z.S.; Wang, K.L.; Karabiyik, B.; Yoshiiri, K.; Rokicka, P.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Noble metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotech. 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Mkhalid, I.A. Visible light photocatalytic synthesis of aniline with an Au/LaTiO3 nanocomposites. J. Alloy Compd. 2015, 631, 298–302. [Google Scholar] [CrossRef]
- Seo, J.H.; Jeon, W.I.; Dembereldorj, U.; Lee, S.Y.; Joo, S.W. Cytotoxicity of serum protein-adsorbed visible-light photocatalytic Ag/AgBr/TiO2 nanoparticles. J. Hazard. Mater. 2011, 198, 347–355. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Fazio, G.; Ferrighi, L.; Di Valentin, C. Spherical versus faceted anatase TiO2 nanoparticles: A model study of structural and electronic properties. J. Phys. Chem. C 2015, 119, 20735–20746. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Wang, K.; Colbeau-Justin, C.; Ohtani, B. Enhanced photocatalytic activity of octahedral anatase particles prepared by hydrothermal reaction. Catal. Today 2017, 280, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Kowalska, E.; Ohtani, B. Influence of post-treatment operations on structural properties and photocatalytic activity of octahedral anatase titania particles prepared by an ultrasonication-hydrothermal reaction. Molecules 2014, 19, 19573–19587. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.A.; Wang, S.H.; Bian, Z.F.; Xie, S.H.; Cai, C.L.; Wang, J.G.; Yang, H.G.; Li, H.X. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant {001} facets and enhanced photocatalytic activity. CrystEngComm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-controlled anatase titanium(IV) oxide particles prepared by hydrothermal treatment of peroxo titanic acid in the presence of polyvinyl alcohol. J. Phys. Chem. C 2009, 113, 3062–3069. [Google Scholar] [CrossRef] [Green Version]
- Ballestas-Barrientos, A.; Li, X.B.; Yick, S.; Yuen, A.; Masters, A.F.; Maschmeyer, T. Interactions of plasmonic silver nanoparticles with high energy sites on multi-faceted rutile TiO2 photoanodes. ChemCatChem 2020, 12, 469–477. [Google Scholar] [CrossRef]
- Li, K.; Peng, T.Y.; Ying, Z.H.; Song, S.S.; Zhang, J. Ag-loading on brookite TiO2 quasi nanocubes with exposed {210} and {001} facets: Activity and selectivity of CO2 photoreduction to CO/CH4. Appl. Catal. B Environ. 2016, 180, 130–138. [Google Scholar] [CrossRef]
- Bryl, R.; Olewicz, T.; de Bocarme, T.V.; Kruse, N. Thermal faceting of clean and oxygen-covered Ir nanocrystals. J. Phys. Chem. C 2011, 115, 2761–2768. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Zhan, B.W.; Ge, B.H.; Zhu, Y.T.; Dai, Y.; Zhou, G.J.; Yu, F.P.; Wang, P.; Huang, B.B.; Zhan, J. Synthesis of high efficient and stable plasmonic photocatalyst Ag/AgNbO3 with specific exposed crystal-facets and intimate heterogeneous interface via combustion route. Appl. Surf. Sci. 2019, 488, 485–493. [Google Scholar] [CrossRef]
- Hou, C.T.; Liu, W.L.; Zhu, J.M. Synthesis of NaOH-modified TiOF2 and its enhanced visible light photocatalytic performance on RhB. Catalysts 2017, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Z.; Kovarik, L.; Mei, D.H.; Liu, J.; Wang, Y.; Peden, C.H.F. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Y.; Dong, X.A.; Sun, Y.J.; Cen, W.L.; Dong, F. Facet-dependent interfacial charge separation and transfer in plasmonic photocatalysts. Appl. Catal. B Environ. 2018, 226, 269–277. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.Z.; Sun, S.M.; Jiang, D.; Gao, E.P. Selective transport of electron and hole among {001} and {110} facets of BiOCl for pure water splitting. Appl. Catal. B Environ. 2015, 162, 470–474. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.Q.; Sun, Y.J.; Zhang, Y.X.; Huang, H.W.; Wu, Z.B. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Warmuth, L.; Ritschel, C.; Feldmann, C. Facet-, composition- and wavelength-dependent photocatalysis of Ag2MoO4. RSC Adv. 2020, 10, 18377–18383. [Google Scholar] [CrossRef]
- Li, R.G.; Han, H.X.; Zhang, F.X.; Wang, D.G.; Li, C. Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4. Energy Environ. Sci. 2014, 7, 1369–1376. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, W.Y.; Miao, Y.Q.; Feng, G.; Zhang, R.B. Facet-selective photodeposition of gold nanoparticles on faceted ZnO crystals for visible light photocatalysis. J. Colloid Interf. Sci. 2016, 475, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, M.C.; Zhou, Z.H.; Guo, L.J. Surface activation of faceted photocatalyst: When metal cocatalyst determines the nature of the facets. Adv. Sci. 2015, 2, 1500153. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase tiania particles as visible light-responsive plasmonic photocatalyst. J. Photonics Energy 2017, 7, 1–16. [Google Scholar]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazi, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Endo, M.; Janczarek, M.; Wei, Z.; Wang, K.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Bactericidal properties of plasmonic photocatalysts composed of noble-metal nanoparticles on faceted ana-tase titania. J. Nanosci. Nanotechnol. 2019, 19, 442–452. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Kowalska, E. Plasmonic photocatalysts for microbiological applications. Catalysts 2020, 10, 824. [Google Scholar] [CrossRef]
- Mao, J.; Ye, L.Q.; Li, K.; Zhang, X.H.; Liu, J.Y.; Peng, T.Y.; Zan, L. Pt-loading reverses the photocatalytic activity order of anatase TiO2 {001} and {010} facets for photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2014, 144, 855–862. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Chen, X.X.; Gong, B.G.; Zhao, Y.C.; Zhang, J.Y.; Zheng, C.G.; Wu, J.C.S. CO2 photocatalytic reduction over Pt deposited TiO2 nanocrystals with coexposed {101} and {001} facets: Effect of deposition method and Pt precursors. Catal. Commun. 2017, 96, 1–5. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takashima, M.; Takase, M.; Ohtani, B. Mechanistic study on facet-dependent deposition of metal nanoparticles on decahedral-shaped anatase titania photocatalyst particles. Catalysts 2018, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- Zielinska-Jurek, A.; Wei, Z.S.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-controlled synthesis of Pt particles on TiO2 surface: Physicochemical characteristic and photocatalytic activity. Catalysts 2019, 9, 940. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, X.J.; Fang, M.L.; Chen, J.Y.; Li, Y.J.; Xie, Z.X.; Kuang, Q.; Zheng, L.S. Photo-induced Au-Pd alloying at TiO2 {101} facets enables robust CO2 photocatalytic reduction into hydrocarbon fuels. J. Mater. Chem. A 2019, 7, 1334–1340. [Google Scholar] [CrossRef]
- Bian, J.; Qu, Y.; Zhang, X.L.; Sun, N.; Tang, D.Y.; Jing, L.Q. Dimension-matched plasmonic Au/TiO2/BiVO4 nanocomposites as efficient wide-visible-light photocatalysts to convert CO2 and mechanistic insights. J. Mater. Chem. A 2018, 6, 11838–11845. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Keulemans, M.; Goris, B.; Blommaerts, N.; Bals, S.; Martens, J.A.; Lenaerts, S. Plasmonic ‘rainbow’ photocatalyst with broadband solar light response for environmental applications. Appl. Catal. B Environ. 2016, 188, 147–153. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodriguez-Lopez, J.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for applications in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Kowalska, E.; Janczarek, M.; Rosa, L.; Juodkazi, S.; Ohtani, B. Mono- and bi-metallic plasmonic photocatalysts for degradation of organic compounds under UV and visible light irradiation. Catal. Today 2014, 230, 131–137. [Google Scholar] [CrossRef]
- Malankowska, A.; Kobylanski, M.P.; Mikolajczyk, A.; Cavdar, O.; Nowaczyk, G.; Jarek, M.; Lisowski, W.; Michalska, M.; Kowalska, E.; Ohtani, B.; et al. TiO2 and NaTaO3 decorated by trimetallic Au/Pd/Pt core-shell nanoparticles as efficient photocatalysts: Experimental and computational studies. ACS Sustain. Chem. Eng. 2018, 6, 16665–16682. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef] [Green Version]
- DeSario, P.A.; Pietron, J.J.; DeVantier, D.E.; Brintlinger, T.H.; Stroud, R.M.; Rolison, D.R. Plasmonic enhancement of visible-light water splitting with Au-TiO2 composite aerogels. Nanoscale 2013, 5, 8073–8083. [Google Scholar] [CrossRef]
- Panayotov, D.A.; DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; Szymczak, L.C.; Rolison, D.R.; Morris, J.R. Ultraviolet and visible photochemistry of methanol at 3D mesoporous networks: TiO2 and Au-TiO2. J. Phys. Chem. C 2013, 117, 15035–15049. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Kanda, T.; Torigoe, K.; Sakai, H.; Abe, M. Preparation of gold/silver/titania trilayered nanorods and their photocatalytic activities. Langmuir 2014, 30, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Bielan, Z.; Kowalska, E.; Dudziak, S.; Wang, K.; Ohtani, B.; Zielinska-Jurek, A. Mono- and bimetallic (Pt/Cu) titanium(IV) oxide core–shell photocatalysts with UV/Vis light activity and magnetic separability. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Bielan, Z.; Sulowska, A.; Dudziak, S.; Siuzdak, K.; Ryl, J.; Zielinska-Jurek, A. Defective TiO2 core-shell magnetic photocatalyst modified with plasmonic nanoparticles for visible light-induced photocatalytic activity. Catalysts 2020, 10, 672. [Google Scholar] [CrossRef]
- Zheng, S.; Wei, Z.; Yoshiiri, K.; Braumuller, M.; Ohtani, B.; Rau, S.; Kowalska, E. Titania modification with ruthenium(II) complex and gold nanoparticles for photocatalytic degradation of organic compounds. Photochem. Photobiol. Sci. 2016, 15, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.Z.; Wang, K.L.; Wei, Z.S.; Yoshiiri, K.; Braumuller, M.; Rau, S.; Ohtani, B.; Kowalska, E. Mono- and dual-modified titania with a ruthenium(II) complex and silver nanoparticles for photocatalytic degradation of organic compounds. J. Adv. Oxid. Technol. 2016, 19, 208–217. [Google Scholar] [CrossRef]
- Kowalska, E.; Yoshiiri, K.; Wei, Z.; Zheng, S.; Kastl, E.; Remita, H.; Ohtani, B.; Rau, S. Hybrid photocatalysts composed of titania modified with plasmonic nanoparticles and ruthenium complexes for decomposition of organic compounds. Appl. Catal. B Environ. 2015, 178, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.M.; Zhou, G.; Mi, J.; Wu, Z.Y. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Synergistic effect in plasmonic Au/Ag alloy NPs co-coated TiO2 NWs toward visible-light enhanced CO2 photoreduction to fuels. Appl. Catal. B Environ. 2017, 204, 548–560. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.R.; Ayub, I.; Wang, S.L.; Yan, W. In situ decoration of g-C3N4 quantum dots on 1D branched TiO2 loaded with plasmonic Au nanoparticles and improved the photocatalytic hydrogen evolution activity. Appl. Surf. Sci. 2020, 519, 146208. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, Z.T. Preparation of the Au@TiO2 nanofibers by one-step electrospinning for the composite photoanode of dye-sensitized solar cells. Mater. Chem. Phys. 2018, 208, 35–40. [Google Scholar] [CrossRef]
- Khatun, F.; Abd Aziz, A.; Sim, L.C.; Monir, M.U. Plasmonic enhanced Au decorated TiO2 nanotube arrays as a visible light active catalyst towards photocatalytic CO2 conversion to CH4. J. Environ. Chem. Eng. 2019, 7, 103233. [Google Scholar] [CrossRef]
- Fu, N.Q.; Jiang, X.Z.; Chen, D.C.; Duan, Y.D.; Zhang, G.G.; Chang, M.L.; Fang, Y.Y.; Lin, Y. Au/TiO2 nanotube array based multi-hierarchical architecture for highly efficient dye-sensitized solar cells. J. Power Sources 2019, 439, 227076. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Huo, W.; Zhu, K.; Zhang, X.; Zhou, X.; Fang, F.; Xie, Z.G.; Jiang, J. Preferentially oriented Ag-TiO2 nanotube array film: An efficient visible-light-driven photocatalyst. J. Hazard. Mater. 2020, 399, 123016. [Google Scholar] [CrossRef]
- Mazierski, P.; Malankowska, A.; Kobylanski, M.; Diak, M.; Kozak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. Photocatalytically active TiO2/Ag2O nanotube arrays interlaced with silver nanoparticles obtained from the one-step anodic oxidation of Ti-Ag alloys. ACS Catal. 2017, 7, 2753–2764. [Google Scholar] [CrossRef]
- Wei, Z.S.; Endo-Kimura, M.; Wang, K.L.; Colbeau-Justin, C.; Kowalska, E. Influence of semiconductor morphology on photocatalytic activity of plasmonic photocatalysts: Titanate nanowires and octahedral anatase nanoparticles. Nanomaterials 2019, 9, 1447. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Q.; Wu, G.J.; Dai, W.L.; Guan, N.J.; Li, L.D. Synthetic design of gold nanoparticles on anatase TiO2 {001} for enhanced visible light harvesting. ACS Sustain. Chem. Eng. 2014, 2, 1940–1946. [Google Scholar] [CrossRef]
- Kumar, N.S.; Asif, M.; Reddy, T.R.K.; Shanmugam, G.; Ajbar, A. Silver quantum dot decorated 2D-SnO2 nanoflakes for photocatalytic degradation of the water pollutant Rhodamine B. Nanomaterials 2019, 9, 1536. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Li, Z.D.; Yu, Y.H.; Ma, Y.J.; Yang, W.G.; Wang, F.; Yin, X.; Wang, X.D. Surface-plasmon-resonance-enhanced photoelectrochemical water splitting from Au-nanoparticle-decorated 3D TiO2 nanorod architectures. J. Phys. Chem. C 2017, 121, 12071–12079. [Google Scholar] [CrossRef]
- Song, C.K.; Baek, J.; Kim, T.Y.; Yu, S.; Han, J.W.; Yi, J. Exploring crystal phase and morphology in the TiO2 supporting materials used for visible-light driven plasmonic photocatalyst. Appl. Catal. B Environ. 2016, 198, 91–99. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Endo, M.; Wang, K.; Bahena, D.; Rodriguez-Lopez, J.L.; Remita, H. Inhibition of fungal growth using modified TiO2 with core@shell structure of Ag@CuO clusters. ACS Appl. Bio Mater. 2019, 2, 5626–5633. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic crystals for plasmonic photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.T.; Chen, S.; Quan, X.; Zhao, H.M. Integrating plasmonic nanoparticles with TiO2 photonic crystal for enhancement of visible-light-driven photocatalysis. Environ. Sci. Technol. 2012, 46, 1724–1730. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Lee, S.T.; Yang, S.H.; Kang, Z.H. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Zhang, S.S.; Peng, B.Y.; Yang, S.Y.; Wang, H.G.; Yu, H.; Fang, Y.P.; Peng, F. Non-noble metal copper nanoparticles-decorated TiO2 nanotube arrays with plasmon-enhanced photocatalytic hydrogen evolution under visible light. Int. J. Hydrogen Energy 2015, 40, 303–310. [Google Scholar] [CrossRef]
- Ebessen, T.W.; Lezec, H.J.; Ghaemi, H.F.; Thio, T.; Wolff, P.A. Extraordinary optical transmission through sub-wavelength hole arrays. Nature 1998, 391, 667669. [Google Scholar] [CrossRef]
- Rodrigo, S.G.; de Leon-Perez, F.; Martin-Moreno, L. Extraordinary optical transmission: Fundamentals and applications. Proc. IEEE 2016, 104, 2288–2306. [Google Scholar] [CrossRef] [Green Version]
- Boppella, R.; Kochuveedu, S.T.; Kim, H.; Jeong, M.J.; Mota, F.M.; Park, J.H.; Kim, D.H. Plasmon-sensitized graphene/TiO2 inverse opal nanostructures with enhanced charge collection efficiency for water splitting. ACS Appl. Mater. Interfaces 2017, 9, 7075–7083. [Google Scholar] [CrossRef]
- Kakekhani, A.; Ismail-Beigi, S.; Altman, E.I. Ferroelectrics: A pathway to switchable surface chemistry and catalysis. Surf. Sci. 2016, 650, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Su, R.; Shen, Y.J.; Li, L.L.; Zhang, D.W.; Yang, G.; Gao, C.B.; Yang, Y.D. Silver-modified nnosized frroelectrics as a nvel potocatalyst. Small 2015, 11, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ding, T.; Wang, Y.; Zhao, W.; Xian, H.; Tian, Y.; Zhang, T.; Li, X. Rational construction of plasmon Au assisted ferroelectric-BaTiO3/Au/g-C3N4 Z-scheme system for efficient photocatalysis. Catal. Today 2019. [Google Scholar] [CrossRef]
- Cui, Y.F.; Sun, H.H.; Shen, G.D.; Jing, P.P.; Pu, Y.P. Effect of dual-cocatalyst surface modification on photodegradation activity, pathway, and mechanisms with highly efficient Ag/BaTiO3/MnOx. Langmuir 2020, 36, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.Y.; Zhou, X.M.; Liu, G.; Yu, J.G.; Zhang, J.C.; Zhi, L.J.; Nie, G.J. Enhancing photocatalytic activity of one-dimensional KNbO3 nanowires by Au nanoparticles under ultraviolet and visible-light. Nanoscale 2011, 3, 5161–5167. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wang, B.; Wang, X.Z.; Xiao, X.H.; Dai, Z.G.; Wu, W.; Zheng, J.F.; Ren, F.; Jiang, C.Z. Preparation of M@BiFeO3 nanocomposites (M = Ag, Au) bowl arrays with enhanced visible light photocatalytic activity. J. Am. Ceram. Soc. 2015, 98, 2255–2263. [Google Scholar] [CrossRef]
- Cui, Y.F.; Briscoe, J.; Dunn, S. Effect of ferroelectricity on solar-light-driven photocatalytic activity of BaTiO3-Influence on the carrier separation and stern layer formation. Chem. Mater. 2013, 25, 4215–4223. [Google Scholar] [CrossRef]
- Cui, Y.F.; Goldup, S.M.; Dunn, S. Photodegradation of Rhodamine B over Ag modified ferroelectric BaTiO3 under simulated solar light: Pathways and mechanism. RSC Adv. 2015, 5, 30372–30379. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.Y.; Zhou, Y.P.; Han, T.L.; Yang, Y.Y.; Wei, J.Y.; Li, H.; He, W.W. Ferroelectric polarization-enhanced photocatalytic properties and photo-induced charge carrier behavior of Au/BaTiO3. J. Alloy. Compd. 2020, 825, 154060. [Google Scholar] [CrossRef]
- Kumar, V.; O’Donnell, S.; Zoellner, B.; Martinez, J.; Wang, G.; Maggard, P.A. Interfacing plasmonic nanoparticles with ferroelectrics for hot-carrier-driven photocatalysis: Impact of Schottky barrier height. ACS Appl. Energy Mater. 2019, 2, 7690–7699. [Google Scholar] [CrossRef]




















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-Governed Performance of Plasmonic Photocatalysts. Catalysts 2020, 10, 1070. https://doi.org/10.3390/catal10091070
Wei Z, Janczarek M, Wang K, Zheng S, Kowalska E. Morphology-Governed Performance of Plasmonic Photocatalysts. Catalysts. 2020; 10(9):1070. https://doi.org/10.3390/catal10091070
Chicago/Turabian StyleWei, Zhishun, Marcin Janczarek, Kunlei Wang, Shuaizhi Zheng, and Ewa Kowalska. 2020. "Morphology-Governed Performance of Plasmonic Photocatalysts" Catalysts 10, no. 9: 1070. https://doi.org/10.3390/catal10091070
APA StyleWei, Z., Janczarek, M., Wang, K., Zheng, S., & Kowalska, E. (2020). Morphology-Governed Performance of Plasmonic Photocatalysts. Catalysts, 10(9), 1070. https://doi.org/10.3390/catal10091070