Noble Metal Modification of CdS-Covered CuInS2 Electrodes for Improved Photoelectrochemical Activity and Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
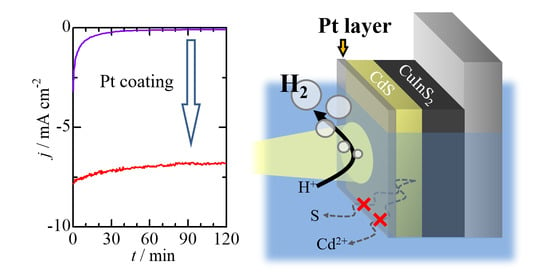
2.2. Photoelectrochemical Measurements
2.3. Photoelectrochemical CO2 Reduction
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of CuInS2 Film on Mo Foil
3.3. Surface Modification of CuInS2 Electrode
3.4. Characterization
3.5. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent Advances and Emerging Trends in Photo-Electrochemical Solar Energy Conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, T.; Gong, J. Single-Crystal Silicon-Based Electrodes for Unbiased Solar Water Splitting: Current Status and Prospects. Chem. Soc. Rev. 2019, 48, 2158–2181. [Google Scholar] [CrossRef]
- Varadhan, P.; Fu, H.C.; Kao, Y.C.; Horng, R.H.; He, J.H. An Efficient and Stable Photoelectrochemical System with 9% Solar-to-Hydrogen Conversion Efficiency via InGaP/GaAs Double Junction. Nat. Commun. 2019, 10, 5282. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, D.; Lumley, M.A.; Choi, K.S. Progress on Ternary Oxide-Based Photoanodes for Use in Photoelectrochemical Cells for Solar Water Splitting. Chem. Soc. Rev. 2019, 48, 2126–2157. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-Dimensional Materials in Semiconductor Photoelectrocatalytic Systems for Water Splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.S. Photoelectrochemical Water Splitting with p-Type Metal Oxide Semiconductor Photocathodes. ChemSusChem 2018, 12, 1835–1845. [Google Scholar] [CrossRef]
- Yang, W.; Moon, J. Recent Advances in Earth-Abundant Photocathodes for Photoelectrochemical Water Splitting. ChemSusChem 2018, 12, 1889–1899. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Nakamura, T.; Lee, S.M.; Yagi, T.; Harada, T.; Minegishi, T.; Matsumura, M. Photoreduction of Water by Using Modified CuInS2 Electrodes. ChemSusChem 2010, 4, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Minegishi, T.; Kaneko, H.; Ma, G.; Zhong, M.; Nakabayashi, M.; Hisatomi, T.; Katayama, M.; Shibata, N.; Yamada, T.; et al. Efficient Hydrogen Evolution on (CuInS2)X(ZnS)1−X Solid Solution-Based Photocathodes under Simulated Sunlight. Chem. Commun. 2019, 55, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hakari, Y.; Ikeda, S.; Jia, Q.; Iwase, A.; Kudo, A. Utilization of Metal Sulfide Material of (CuGa)1−XZn2XS2 Solid Solution with Visible Light Response in Photocatalytic and Photoelectrochemical Solar Water Splitting Systems. J. Phys. Chem. Lett. 2015, 6, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Septina, W.; Gunawan; Ikeda, S.; Harada, T.; Higashi, M.; Abe, R.; Matsumura, M. Photosplitting of Water from Wide-Gap Cu(In,Ga)S2 Thin Films Modified with a CdS Layer and Pt Nanoparticles for a High-Onset-Potential Photocathode. J. Phys. Chem. C 2015, 119, 8576–8583. [Google Scholar] [CrossRef]
- Guijarro, N.; Prévot, M.S.; Yu, X.; Jeanbourquin, X.A.; Bornoz, P.; Bourée, W.; Johnson, M.; Le Formal, F.; Sivula, K. A Bottom-up Approach toward All-Solution-Processed High-Efficiency Cu(In,Ga)S2 Photocathodes for Solar Water Splitting. Adv. Energy Mater. 2016, 6, 1501949–1501961. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Maeda, K.; Katayama, M.; Kubota, J.; Yamada, A.; Konagai, M.; Domen, K. Photoelectrochemical Water Splitting Using a Cu(In,Ga)Se2 Thin Film. Electrochem. Commun. 2010, 12, 851–853. [Google Scholar] [CrossRef]
- Moriya, M.; Minegishi, T.; Kumagai, H.; Katayama, M.; Kubota, J.; Domen, K. Stable Hydrogen Evolution from CdS-Modified CuGaSe2 Photoelectrode under Visible-Light Irradiation. J. Am. Chem. Soc. 2013, 135, 3733–3735. [Google Scholar] [CrossRef]
- Septina, W.; Ikeda, S.; Harada, T.; Minegishi, T.; Domen, K.; Matsumura, M. Platinum and Indium Sulfide-Modified Cuins2 as Efficient Photocathodes for Photoelectrochemical Water Splitting. Chem. Commun. 2014, 50, 8941–8943. [Google Scholar]
- Septina, W.; Harada, T.; Nose, Y.; Ikeda, S. Investigation of the Electric Structures of Heterointerfaces in Pt- and In2S3-Modified CuInS2 Photocathodes Used for Sunlight-Induced Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 16086–16092. [Google Scholar]
- Chae, S.Y.; Park, S.J.; Han, S.G.; Jung, H.; Kim, C.W.; Jeong, C.; Joo, O.S.; Min, B.K.; Hwang, Y.J. Enhanced Photocurrents with ZnS Passivated Cu(In,Ga)(Se,S)2 Photocathodes Synthesized Using a Nonvacuum Process for Solar Water Splitting. J. Am. Chem. Soc. 2016, 138, 15673–15681. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shavel, A.; An, X.; Luo, Z.; Ibánez, M.; Cabot, A. Cu2ZnSnS4-Pt and Cu2ZnSnS4-Au Heterostructured Nanoparticles for Photocatalytic Water Splitting and Pollutant Degradation. J. Am. Chem. Soc. 2014, 136, 9236–9239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ruan, Y.; Feng, S.; Chen, S.; Su, K. Ag Clusters Anchored Conducting Polyaniline as Highly Efficient Cocatalyst for Cu2ZnSnS4 Nanocrystals toward Enhanced Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2018, 6, 11424–11432. [Google Scholar] [CrossRef]
- Osaki, J.; Yoda, M.; Takashima, T.; Irie, H. Selective Loading of Platinum or Silver Cocatalyst onto a Hydrogen-Evolution Photocatalyst in a Silver-Mediated All Solid-State Z-Scheme System for Enhanced Overall Water Splitting. RSC Adv. 2019, 9, 41913–41917. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.M.; Saeki, S.; Sekizawa, K.; Kitazumi, K.; Takahashi, N.; Morikawa, T. Photoelectrochemical Hydrogen Production by Water Splitting over Dual-Functionally Modified Oxide: P-Type N-Doped Ta2O5 Photocathode Active under Visible Light Irradiation. Appl. Catal. B 2017, 202, 597–604. [Google Scholar] [CrossRef]
- Li, Y.; Luo, K. Performance Improvement of p-Cu2O Nanocrystal Photocathode with Ultra-Thin Silver Protective Layer. Chem. Commun. 2019, 55, 9963–9966. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly Active Oxide Photocathode for Photoelectrochemical Water Reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Zhao, J.; Minegishi, T.; Zhang, L.; Zhong, M.; Nakabayashi, M.; Ma, G.; Hisatomi, T.; Katayama, M.; Ikeda, S.; Shibata, N.; et al. Enhancement of Solar Hydrogen Evolution from Water by Surface Modification with CdS and TiO2 on Porous CuInS2 Photocathodes Prepared by an Electrodeposition—Sulfurization Method. Angew. Chem. Int. Ed. 2014, 53, 11808–11812. [Google Scholar] [CrossRef]
- Mali, M.G.; Yoon, H.; Joshi, B.N.; Park, H.; Al-Deyab, S.S.; Lim, D.C.; Ahn, S.; Nervi, C.; Yoon, S.S. Enhanced Photoelectrochemical Solar Water Splitting Using a Platinum-Decorated CIGS/CdS/ZnO Photocathode. ACS Appl. Mater. Interfaces 2015, 7, 21619–21625. [Google Scholar] [CrossRef]
- Koo, B.; Nam, S.W.; Haight, R.; Kim, S.; Oh, S.; Cho, M.; Oh, J.; Lee, J.Y.; Ahn, B.T.; Shin, B. Tailoring Photoelectrochemical Performance and Stability of Cu(In,Ga)Se2 Photocathode via TiO2-Coupled Buffer Layers. ACS Appl. Mater. Interfaces 2017, 9, 5279–5287. [Google Scholar] [CrossRef]
- Lee, S.M.; Ikeda, S.; Otsuka, Y.; Septina, W.; Harada, T.; Matsumura, M. Homogeneous Electrochemical Deposition of In on a Cu-Covered Mo Substrate for Fabrication of Efficient Solar Cells with a CuInS2 Photoabsorber. Electrochim. Acta 2012, 79, 189–196. [Google Scholar] [CrossRef]
- Matoba, K.; Matsuda, Y.; Takahashi, M.; Sakata, Y.; Zhang, J.; Higashimoto, S. Fabrication of Pt/In2S3/CuInS2 Thin Film as Stable Photoelectrode for Water Splitting under Solar Light Irradiation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Takashima, T.; Sano, T.; Irie, H. Cocatalyst Modification of Niobium-Substituted Silver Tantalate Photocatalyst for Enhanced Solar Water-Splitting Activity. Int. J. Hydrogen Energy 2019, 44, 23600–23609. [Google Scholar] [CrossRef]
- Takashima, T.; Sano, T.; Irie, H. Improvement of The Photocatalytic Water Splitting Activity of Silver Tantalate by Photodeposited Platinum and Cobalt-Oxide Nanoclusters. Electrochemistry 2016, 84, 784–788. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Minegishi, T.; Higashi, T.; Nakabayashi, M.; Shibata, N.; Domen, K. Stable Hydrogen Production from Water on an NIR-Responsive Photocathode under Harsh Conditions. Small 2018, 2, 1800018. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, C.; Zhu, M.; Fu, Y.; Huang, H.; Liu, Y.; Kang, Z. Highly Selective and Efficient Electroreduction of Carbon Dioxide to Carbon Monoxide with Phosphate Silver-Derived Coral-like Silver. ACS Sustain. Chem. Eng. 2019, 7, 3536–3543. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 reduction on metal electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–189. [Google Scholar]
- Ikeda, S.; Fujikawa, S.; Harada, T.; Nguyen, T.H.; Nakanishi, S.; Takayama, T.; Iwase, A.; Kudo, A. Photocathode Characteristics of a Spray-Deposited Cu2ZnGeS4 Thin Film for CO2 Reduction in a CO2-Saturated Aqueous Solution. ACS Appl. Energy Mater. 2019, 2, 6911–6918. [Google Scholar] [CrossRef]
- Ikeda, S.; Tanaka, Y.; Kawaguchi, T.; Fujikawa, S.; Harada, T.; Takayama, T.; Iwase, A.; Kudo, A. Photoelectrochemical Reduction of CO2 to CO Using a CuGaS2 Thin-film Photocathode Prepared by a Spray Pyrolysis Method. Chem. Lett. 2018, 47, 1424–1427. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, S.; Sasaki, Y.; Kanaya, M.; Tsubota, T.; Ohno, T. Improvement of Selectivity for CO2 Reduction by Using Cu2ZnSnS4 Electrodes Modified with Different Buffer Layers (CdS and In2S3) under Visible Light Irradiation. RSC Adv. 2016, 6, 112594–112601. [Google Scholar] [CrossRef]
- Nesbitt, N.T.; Ma, M.; Trześniewski, B.J.; Jaszewski, S.; Tafti, F.; Burns, M.J.; Smith, W.A.; Naughton, M.J. Au Dendrite Electrocatalysts for CO2 Electrolysis. J. Phys. Chem. C 2018, 122, 10006–10016. [Google Scholar] [CrossRef]
- Urbain, F.; Tang, P.; Carretero, N.M.; Andreu, T.; Arbiol, J.; Morante, J.R. Tailoring Copper Foam with Silver Dendrite Catalysts for Highly Selective Carbon Dioxide Conversion into Carbon Monoxide. ACS Appl. Mater. Interfaces 2018, 10, 43650–43660. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagaraja, C.M. Template-Free Synthesis of Zn1-XCdXS Nanocrystals with Tunable Band Structure for Efficient Water Splitting and Reduction of Nitroaromatics in Water. ACS Sustain. Chem. Eng. 2017, 5, 4293–4303. [Google Scholar] [CrossRef]
- Hernández-Calderón, V.; Vigil-Galán, O.; Pulgarín-Agudelo, F.A.; Courel, M.; Arce-Plaza, A.; Cruz-Gandarilla, F.; Sánchez-Rodríguez, F.J.; Roque-De la Puente, J. Optimization of CdXZn1-XS Compound from CdS/ZnS Bi-Layers Deposited by Chemical Bath Deposition for Thin Film Solar Cells Application. Thin Solid Films 2019, 676, 100–107. [Google Scholar] [CrossRef]








| Photoelectrode | j (mA/cm2 at −0.11 V) | Faradaic Efficiency (%) | |
|---|---|---|---|
| H2 | CO | ||
| Pt0.88/CdS/CuInS2 | 4.0 | 99 | 0.36 |
| Au/CdS/CuInS2 | 1.5 | 93 | 6.7 |
| Ag/CdS/CuInS2 | 0.21 | 69 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takashima, T.; Fujishiro, Y.; Irie, H. Noble Metal Modification of CdS-Covered CuInS2 Electrodes for Improved Photoelectrochemical Activity and Stability. Catalysts 2020, 10, 949. https://doi.org/10.3390/catal10090949
Takashima T, Fujishiro Y, Irie H. Noble Metal Modification of CdS-Covered CuInS2 Electrodes for Improved Photoelectrochemical Activity and Stability. Catalysts. 2020; 10(9):949. https://doi.org/10.3390/catal10090949
Chicago/Turabian StyleTakashima, Toshihiro, Yukitaka Fujishiro, and Hiroshi Irie. 2020. "Noble Metal Modification of CdS-Covered CuInS2 Electrodes for Improved Photoelectrochemical Activity and Stability" Catalysts 10, no. 9: 949. https://doi.org/10.3390/catal10090949
APA StyleTakashima, T., Fujishiro, Y., & Irie, H. (2020). Noble Metal Modification of CdS-Covered CuInS2 Electrodes for Improved Photoelectrochemical Activity and Stability. Catalysts, 10(9), 949. https://doi.org/10.3390/catal10090949