Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity
Abstract
:1. Introduction
2. Results
2.1. Total Metal Concentration CTM
2.2. Flow Rate of the Precursor Solution FRPS
2.3. Flow Rate of the Dispersion Gas FRDG
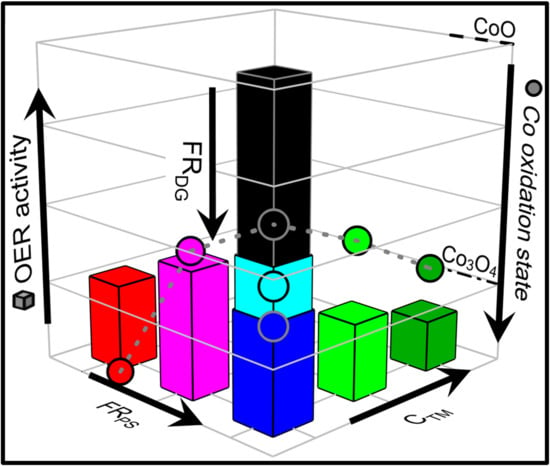
3. Discussion
4. Materials and Methods
4.1. Material Synthesis
4.2. Material Characterization
4.3. Electrochemical Characterisation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bp Statistical Review of World Energy 2019, 68th ed. Available online: https://www.Bp.Com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.Pdf (accessed on 1 February 2020).
- Bockris, J.O. A hydrogen economy. Science 1972, 176, 1323. [Google Scholar] [CrossRef] [PubMed]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo, M.; Fritz, D.L.; Merge, J.; Stolten, D. A comprehensive review on pem water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Fabbri, E.; Schmidt, T.J. Oxygen evolution reaction-the enigma in water electrolysis. ACS Catal. 2018, 8, 9765–9774. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kotz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.J.; Durst, J.; Bozza, F.; Graule, T.; Schaublin, R.; Wiles, L.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheng, X.; Abbott, D.F.; Fabbri, E.; Bozza, F.; Graule, T.; Castelli, I.E.; Wiles, L.; Danilovic, N.; Ayers, K.E.; et al. Highly active nanoperovskite catalysts for oxygen evolution reaction: Insights into activity and stability of Ba0.5Sr0.5Co0.8Fe0.2O2+delta and PrBaCo2O5+delta. Adv. Funct. Mater. 2018, 28, 1804355. [Google Scholar] [CrossRef]
- Kim, B.J.; Fabbri, E.; Abbott, D.F.; Cheng, X.; Clark, A.H.; Nachtegaal, M.; Borlaf, M.; Castelli, I.E.; Graule, T.; Schmidt, T.J. Functional role of fe-doping in co-based perovskite oxide catalysts for oxygen evolution reaction. J. Am. Chem. Soc. 2019, 141, 5231–5240. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Nachtegaal, M.; Cheng, X.; Schmidt, T.J. Superior bifunctional electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3-/carbon composite electrodes: Insight into the local electronic structure. Adv. Energy Mater. 2015, 5, 1402033. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Kim, B.; Nachtegaal, M.; Schmidt, T.J. Effect of ball milling on the electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3 towards the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 13130–13137. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Yamashita, Y.; Castelli, I.E.; Kim, B.; Uchida, M.; Haumont, R.; Puente-Orench, I.; Schmidt, T.J. Oxygen evolution reaction on perovskites: A multieffect descriptor study combining experimental and theoretical methods. ACS Catal. 2018, 8, 9567–9578. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, S.E. Aerosol-based technologies in nanoscale manufacturing: From functional materials to devices through core chemical engineering. AIChE J. 2010, 56, 3028–3035. [Google Scholar] [CrossRef]
- Grohn, A.J.; Pratsinis, S.E.; Sanchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of nanoparticle synthesis by flame spray pyrolysis: The high-temperature particle residence time. Ind. Eng. Chem. Res. 2014, 53, 10734–10742. [Google Scholar] [CrossRef]
- Kühner, G.; Voll, M. Manufacture of carbon black. In Carbon Black Science and Technology, 2nd ed.; Donnet, J.-B., Ed.; Taylor & Francis Group: New York, NY, USA, 1993; Chapter 1; pp. 1–66. [Google Scholar]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently functionalized cobalt nanoparticles as a platform for magnetic separations in organic synthesis. Angew. Chem. Int. Ed. 2007, 46, 4909–4912. [Google Scholar] [CrossRef]
- Turbobeads. Available online: http://www.Turbobeads.Com/ (accessed on 10 February 2020).
- Shao, Z.P.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kotz, R.; Schmidt, T.J. Composite electrode boosts the activity of Ba0.5Sr0.5Co0.8Fe0.2O3-Delta perovskite and carbon toward oxygen reduction in alkaline media. ACS Catal. 2014, 4, 1061–1070. [Google Scholar] [CrossRef]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kotz, R.; Schmidt, T.J. Ba0.5Sr0.5Co0.8Fe0.2O3-Delta perovskite activity towards the oxygen reduction reaction in alkaline media. Chemelectrochem 2014, 1, 338–342. [Google Scholar] [CrossRef]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kotz, R.; Schmidt, T.J. Unraveling the oxygen reduction reaction mechanism and activity of d-band perovskite electrocatalysts for low temperature alkaline fuel cells. ECS Trans. 2014, 64, 1081–1093. [Google Scholar] [CrossRef]
- Mohamed, R.; Fabbri, E.; Levecque, P.; Kotz, R.; Schmidt, T.J.; Conrad, O. Understanding the influence of carbon on the oxygen reduction and evolution activities of bscf/carbon composite electrodes in alkaline electrolyte. ECS Trans. 2014, 58, 9–18. [Google Scholar] [CrossRef]
- Cheng, X.; Kim, B.J.; Fabbri, E.; Schmidt, T.J. Co/Fe oxyhydroxides supported on perovskite oxides as oxygen evolution reaction catalyst systems. Acs Appl. Mater. Interfaces 2019, 11, 34787–34795. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Heel, A.; Holtappels, P.; Hug, P.; Graule, T. Flame spray synthesis of nanoscale La0.6Sr0.4Co0.2Fe0.8O3-δ and Ba0.5Sr0.5Co0.8Fe0.2O3-δ as cathode materials for intermediate temperature solid oxide fuel cells. Fuel Cells 2010, 10, 419–432. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Effect of solvent composition on oxide morphology during flame spray pyrolysis of metal nitrates. Phys. Chem. Chem. Phys. 2011, 13, 9246–9252. [Google Scholar] [CrossRef]
- Wegner, K.; Schimmoeller, B.; Thiebaut, B.; Fernandez, C.; Rao, T.N. Pilot plants for industrial nanoparticle production by flame spray pyrolysis. Kona Powder Part J. 2011, 29, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Mueller, R.; Madler, L.; Pratsinis, S.E. Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem. Eng. Sci. 2003, 58, 1969–1976. [Google Scholar] [CrossRef]
- Mueller, R.; Jossen, R.; Pratsinis, S.E.; Watson, M.; Akhtar, M.K. Zirconia nanoparticles made in spray flames at high production rates. J. Am. Ceram. Soc. 2004, 87, 197–202. [Google Scholar] [CrossRef]
- Grohn, A.J.; Pratsinis, S.E.; Wegner, K. Fluid-particle dynamics during combustion spray aerosol synthesis of zro2. Chem. Eng. J. 2012, 191, 491–502. [Google Scholar] [CrossRef]
- Wegner, K.; Pratsinis, S.E. Scale-up of nanoparticle synthesis in diffusion flame reactors. Chem. Eng. Sci. 2003, 58, 4581–4589. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic powder synthesis by spray-pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. Sci. 1998, 24, 197–219. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Lin, F.Y.; Chang, T.H.; Yeh, C.T. Thermal decomposition of metal nitrates in air and hydrogen environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Kim, B.J.; Fabbri, E.; Castelli, I.E.; Borlaf, M.; Graule, T.; Nachtegaal, M.; Schmidt, T.J. Fe-doping in double perovskite PrBaCo2(1-x)Fe2xO6-: Insights into structural and electronic effects to enhance oxygen evolution catalyst stability. Catalysts 2019, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.P.; Yang, W.S.; Cong, Y.; Dong, H.; Tong, J.H.; Xiong, G.X. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3-Delta oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- McIntosh, S.; Vente, J.F.; Haije, W.G.; Blank, D.H.A.; Bouwmeester, H.J.M. Oxygen stoichiometry and chemical expansion of ba0.5sr0.5co0.8fe0.2o3-delta measured by in situ neutron diffraction. Chem. Mater. 2006, 18, 2187–2193. [Google Scholar] [CrossRef]
- McIntosh, S.; Vente, J.F.; Haije, W.G.; Blank, D.H.A.; Bouwmeester, H.J.M. Structure and oxygen stoichiometry of SrCo0.8Fe0.2O3-Delta and Ba0.5Sr0.5Co0.8Fe0.2O3-Delta. Solid State Ion. 2006, 177, 1737–1742. [Google Scholar] [CrossRef]
- Svarcova, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.M.; Grande, T. Structural instability of cubic perovskite BaxSrxSr1-xCo1-yFeyO3-Delta. Solid State Ion. 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Mastrikov, Y.A.; Kuklja, M.M.; Merkle, R.; Roytburd, A.; Maier, J. First principles calculations of oxygen vacancy formation and migration in mixed conducting Ba0.5Sr0.5Co1-yFeyO3-Delta perovskites. Solid State Ion. 2011, 188, 1–5. [Google Scholar] [CrossRef]
- Yang, Z.; Harvey, A.S.; Infortuna, A.; Schoonman, J.; Gauckler, L.J. Electrical conductivity and defect chemistry of Ba(x)Sr(1-x)CoyFe(1-y)O(3-Delta) perovskites. J. Solid State Electr. 2011, 15, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.S.; Litterst, F.J.; Yang, Z.; Rupp, J.L.M.; Infortuna, A.; Gauckler, L.J. Oxidation states of co and Fe in Ba1-xSrxCo1-yFeyO3-Delta (x, y=0.2–0.8) and oxygen desorption in the temperature range 300–1273 k. Phys. Chem. Chem. Phys. 2009, 11, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.C.; Darriet, J.; Pouchard, M.; Hagenmuller, P. Presentation of new family of perovskite type phases with vacancy ordering for formula a3m308 (amo2.67). Mater. Res. Bull. 1976, 11, 1219–1226. [Google Scholar] [CrossRef]
- Becerro, A.I.; McCammon, C.; Langenhorst, F.; Seifert, F.; Angel, R. Oxygen vacancy ordering in catio3-cafeo2.5 perovskites: From isolated defects to infinite sheets. Phase. Transit. 1999, 69, 133–146. [Google Scholar] [CrossRef]
- Scott, J.F.; Dawber, M. Oxygen-vacancy ordering as a fatigue mechanism in perovskite ferroelectrics. Appl. Phys. Lett. 2000, 76, 3801–3803. [Google Scholar] [CrossRef]
- Eichel, R.A. Structural and dynamic properties of oxygen vacancies in perovskite oxides-analysis of defect chemistry by modern multi-frequency and pulsed epr techniques. Phys. Chem. Chem. Phys. 2011, 13, 368–384. [Google Scholar] [CrossRef]
- Muller, O.; Nachtegaal, M.; Just, J.; Lutzenkirchen-Hecht, D.; Frahm, R. Quick-exafs setup at the superxas beamline for in situ X-ray absorption spectroscopy with 10 ms time resolution. J. Synchrotron Radiat. 2016, 23, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.H.; Imbao, J.; Frahm, R.; Nachtegaal, M. Proqexafs: A highly optimized parallelized rapid processing software for qexafs data. J. Synchrotron Radiat. 2020, 27, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.J.; Gasteiger, H.A.; Stab, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of high-surface area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Shao-Horn, Y. Electrocatalytic measurement methodology of oxide catalysts using a thin-film rotating disk electrode. J. Electrochem. Soc. 2010, 157, B1263–B1268. [Google Scholar] [CrossRef]





| Synthesis Parameters | 2.1. CTM | 2.2. FRPS | 2.3. FRDG |
|---|---|---|---|
| CTM/M | 0.1 1, 0.2 or 0.3 | 0.1 | 0.1 |
| FRPS/mL·min−1 | 50 | 50 1, 30 or 10 | 50 |
| FRDG/L·min−1 | 25 | 25 | 25 1, 35 or 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aegerter, D.; Borlaf, M.; Fabbri, E.; Clark, A.H.; Nachtegaal, M.; Graule, T.; Schmidt, T.J. Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity. Catalysts 2020, 10, 984. https://doi.org/10.3390/catal10090984
Aegerter D, Borlaf M, Fabbri E, Clark AH, Nachtegaal M, Graule T, Schmidt TJ. Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity. Catalysts. 2020; 10(9):984. https://doi.org/10.3390/catal10090984
Chicago/Turabian StyleAegerter, Dino, Mario Borlaf, Emiliana Fabbri, Adam H. Clark, Maarten Nachtegaal, Thomas Graule, and Thomas J. Schmidt. 2020. "Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity" Catalysts 10, no. 9: 984. https://doi.org/10.3390/catal10090984
APA StyleAegerter, D., Borlaf, M., Fabbri, E., Clark, A. H., Nachtegaal, M., Graule, T., & Schmidt, T. J. (2020). Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity. Catalysts, 10(9), 984. https://doi.org/10.3390/catal10090984