Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx
Abstract
:1. Introduction
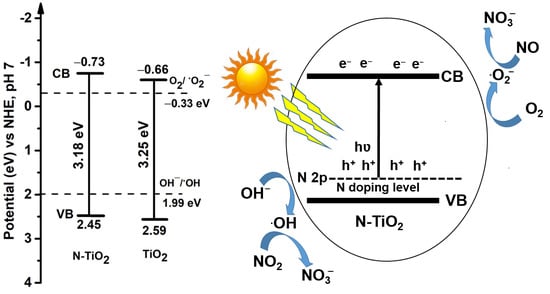
2. Results and Discussion
VVB = VCB + Eg/e,
3. Materials and Methods
3.1. Preparation of N-Doped TiO2
3.2. Characterization
3.3. Electrochemical Measurements
3.4. Photocatalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkanna, R.; Nikhil, G.; Rao, T.S.; Sinha, P.; Swamy, Y. Environmental monitoring of surface ozone and other trace gases over different time scales: Chemistry, transport and modeling. Int. J. Environ. Sci. Technol. 2015, 12, 1749–1758. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, S.; Liu, H.; Xu, J.; Fu, K.; Klimont, Z.; Hao, J.; He, K.; Cofala, J.; Amann, M. NOx emissions in China: Historical trends and future perspectives. Atmos. Chem. Phys. 2013, 13, 9869–9897. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Wang, Y.; Ma, Q.; Ma, J.; Chu, B.; Ji, D.; Tang, G.; Liu, C.; Zhang, H.; Hao, J. Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days. Sci. Rep. 2014, 4, 4172. [Google Scholar] [CrossRef] [PubMed]
- Kotchmar, D.J.; Garner, J.; Gardner, D.; Comfort, B. Air Quality Criteria for Oxides of Nitrogen; Diane Publishing: Darby, PA, USA, 1996. [Google Scholar]
- Lebowitz, M.D.; Walkinshaw, D.S. Indoor Air’90: Health effects associated with indoor air contaminants. Arch. Environ. Health Int. J. 1992, 47, 6–7. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, J.; Lu, G.; Tang, Z. Controlled Synthesis of TiO2 Shape and Effect on the Catalytic Performance for Selective Catalytic Reduction of NO x with NH3. Catal. Surv. Asia 2018, 22, 105–117. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Oskooei, A.B.; Koohsorkhi, J.; Mehrpooya, M. Simulation of plasma-assisted catalytic reduction of NOx, CO, and HC from diesel engine exhaust with COMSOL. Chem. Eng. Sci. 2019, 197, 135–149. [Google Scholar] [CrossRef]
- Yu, Q.; Brouwers, H. Indoor air purification using heterogeneous photocatalytic oxidation. Part I: Experimental study. Appl. Catal. B Environ. 2009, 92, 454–461. [Google Scholar] [CrossRef]
- Schwartz-Narbonne, H.; Jones, S.H.; Donaldson, D.J. Indoor lighting releases gas phase nitrogen oxides from indoor painted surfaces. Environ. Sci. Technol. Lett. 2019, 6, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Hakki, A.; Zheng, L.; Jones, M.R.; Wang, F.; Macphee, D.E. Photocatalytic concrete for NOx abatement: Supported TiO2 efficiencies and impacts. Cem. Concr. Res. 2019, 116, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Long, R. Electronic structure of semiconducting and metallic tubes in TiO2/carbon nanotube heterojunctions: Density functional theory calculations. J. Phys. Chem. Lett. 2013, 4, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, Y.; He, G.; Lin, C.; Wei, C. Photocatalytic oxidation of nitrogen oxides over {001} TiO2: The influence of F− ions. Environ. Sci. Pollut. Res. 2018, 25, 35342–35351. [Google Scholar] [CrossRef] [PubMed]
- Maggos, T.; Bartzis, J.; Leva, P.; Kotzias, D. Application of photocatalytic technology for NOx removal. Appl. Phys. A 2007, 89, 81–84. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Liu, Y.; Gu, Z. Photocatalytic oxidation of nitric oxide with immobilized titanium dioxide films synthesized by hydrothermal method. J. Hazard. Mater. 2008, 151, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, D.; Li, R.; Chen, P.; Zhang, Q.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kuo, C.-S.; Huang, C.-H.; Li, Y.-Y.; Chou, P.-W.; Cheng, C.-L.; Wong, M.-S. Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnology 2006, 17, 2490. [Google Scholar] [CrossRef]
- Todorova, N.; Vaimakis, T.; Petrakis, D.; Hishita, S.; Boukos, N.; Giannakopoulou, T.; Giannouri, M.; Antiohos, S.; Papageorgiou, D.; Chaniotakis, E. N and N, S-doped TiO2 photocatalysts and their activity in NOx oxidation. Catal. Today 2013, 209, 41–46. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, X.; Zhao, L.; Sun, M. Photocatalytic oxidation of NOx over visible-light-responsive nitrogen-doped TiO2. Korean J. Chem. Eng. 2007, 24, 1017–1021. [Google Scholar] [CrossRef]
- Todorova, N.; Giannakopoulou, T.; Pomoni, K.; Yu, J.; Vaimakis, T.; Trapalis, C. Photocatalytic NOx oxidation over modified ZnO/TiO2 thin films. Catal. Today 2015, 252, 41–46. [Google Scholar] [CrossRef]
- Abramović, B.F.; Šojić, D.V.; Anderluh, V.B.; Abazović, N.D.; Čomor, M.I. Nitrogen-doped TiO2 suspensions in photocatalytic degradation of mecoprop and (4-chloro-2-methylphenoxy) acetic acid herbicides using various light sources. Desalination 2009, 244, 293–302. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Tian, B.; Chen, F.; Wang, L. Synthesis and characterization of thermally stable Sm, N co-doped TiO2 with highly visible light activity. J. Hazard. Mater. 2010, 182, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, Y.; Liu, X.; Wang, W.; Zhan, P.; Li, Z.; Zhang, Z. Visible light photoelectrochemical properties of N-doped TiO2 nanorod arrays from TiN. J. Nanomater. 2013, 2013, 930950. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Zhang, S.; Wei, S.-H. Origin of p-type doping difficulty in ZnO: The impurity perspective. Phys. Rev. B 2002, 66, 073202. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, R.; Abe, S. Visible-light sensitization of TiO2 photocatalysts by wet-method N doping. Appl. Catal. A Gen. 2005, 284, 131–137. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Hsu, H.-J.; Lin, Y.-H.; Shiesh, C.-C. The synergistic effect of nitrogen dopant and calcination temperature on the visible-light-induced photoactivity of N-doped TiO2. Int. J. Photoenergy 2013, 2013, 268723. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Jun, Y.S.; Hong, W.H.; Antonietti, M.; Thomas, A. Mesoporous, 2D hexagonal carbon nitride and titanium nitride/carbon composites. Adv. Mater. 2009, 21, 4270–4274. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Hossain, M.K.; Pervez, M.; Mia, M.; Tayyaba, S.; Uddin, M.J.; Ahamed, R.; Khan, R.A.; Hoq, M.; Khan, M.A.; Ahmed, F. Annealing temperature effect on structural, morphological and optical parameters of mesoporous TiO2 film photoanode for dye-sensitized solar cell application. Mater. Sci.-Pol. 2018, 35, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Gao, P.; Yang, Y.; Yang, P.; Chen, Y.; Wang, Y. N-doped TiO2 nanobelts with coexposed (001) and (101) facets and their highly efficient visible-light-driven photocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2016, 8, 18126–18131. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2020, 384, 123245. [Google Scholar] [CrossRef]
- Hendarsa, A.; Mustofa, S. Photodegradation of methylcyclohexane in two phases with modified-titania immobilized on pumice. Int. J. Technol. 2017, 8, 27–36. [Google Scholar]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y. The stable and uniform characteristics of nitrogen in nitrogen doped titanium dioxide nano photocatalytic particles. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2009; pp. 1–5. [Google Scholar]
- Ji, T.; Liu, Q.; Zou, R.; Zhang, Y.; Wang, L.; Sang, L.; Liao, M.; Hu, J. Enhanced UV-visible light photodetectors with a TiO2/Si heterojunction using band engineering. J. Mater. Chem. C 2017, 5, 12848–12856. [Google Scholar]
- Batalović, K.; Bundaleski, N.; Radaković, J.; Abazović, N.; Mitrić, M.; Silva, R.; Savić, M.; Belošević-Čavor, J.; Rakočević, Z.; Rangel, C. Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)–a combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q.; Cheng, H.-M. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Xie, G.; Zhang, M.; Hou, Z.; He, Z. Gd3+, N-codoped trititanate nanotubes: Preparation, characterization and photocatalytic activity. Appl. Surf. Sci. 2011, 257, 3728–3732. [Google Scholar] [CrossRef]
- Preclíková, J.; Galář, P.; Trojánek, F.; Daniš, S.; Rezek, B.; Gregora, I.; Němcová, Y.; Malý, P. Nanocrystalline titanium dioxide films: Influence of ambient conditions on surface-and volume-related photoluminescence. J. Appl. Phys. 2010, 108, 113502. [Google Scholar] [CrossRef]
- Wang, E.; He, T.; Zhao, L.; Chen, Y.; Cao, Y. Improved visible light photocatalytic activity of titania doped with tin and nitrogen. J. Mater. Chem. 2011, 21, 144–150. [Google Scholar] [CrossRef]
- Dong, G.; Ho, W.; Li, Y.; Zhang, L. Facile synthesis of porous graphene-like carbon nitride (C6N9H3) with excellent photocatalytic activity for NO removal. Appl. Catal. B Environ. 2015, 174, 477–485. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Beranek, R. (Photo) electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef] [Green Version]
- Spadavecchia, F.; Cappelletti, G.; Ardizzone, S.; Ceotto, M.; Falciola, L. Electronic structure of pure and N-doped TiO2 nanocrystals by electrochemical experiments and first principles calculations. J. Phys. Chem. C 2011, 115, 6381–6391. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Hu, Y.; Zheng, M.; Wei, C. Solvent-free in situ synthesis of g-C3N4/{0 0 1} TiO2 composite with enhanced UV-and visible-light photocatalytic activity for NO oxidation. Appl. Catal. B Environ. 2016, 182, 587–597. [Google Scholar] [CrossRef]
- Hossain, S.M.; Park, H.; Kang, H.-J.; Mun, J.S.; Tijing, L.; Rhee, I.; Kim, J.-H.; Jun, Y.-S.; Shon, H.K. Facile synthesis and characterization of anatase TiO2/g-CN composites for enhanced photoactivity under UV–visible spectrum. Chemosphere 2020, 262, 128004. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Yu, J.; Dimotikali, D.; Trapalis, C. Photocatalytic activity of modified g-C3N4/TiO2 nanocomposites for NOx removal. Catal. Today 2017, 280, 37–44. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.T.; Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. https://doi.org/10.3390/catal11010109
Khan TT, Bari GAKMR, Kang H-J, Lee T-G, Park J-W, Hwang HJ, Hossain SM, Mun JS, Suzuki N, Fujishima A, et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts. 2021; 11(1):109. https://doi.org/10.3390/catal11010109
Chicago/Turabian StyleKhan, Tamal Tahsin, Gazi A. K. M. Rafiqul Bari, Hui-Ju Kang, Tae-Gyu Lee, Jae-Woo Park, Hyun Jin Hwang, Sayed Mukit Hossain, Jong Seok Mun, Norihiro Suzuki, Akira Fujishima, and et al. 2021. "Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx" Catalysts 11, no. 1: 109. https://doi.org/10.3390/catal11010109
APA StyleKhan, T. T., Bari, G. A. K. M. R., Kang, H. -J., Lee, T. -G., Park, J. -W., Hwang, H. J., Hossain, S. M., Mun, J. S., Suzuki, N., Fujishima, A., Kim, J. -H., Shon, H. K., & Jun, Y. -S. (2021). Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts, 11(1), 109. https://doi.org/10.3390/catal11010109