Photocatalytic Properties of Core-Shell Structured Wool-TiO2 Hybrid Composite Powders
Abstract
:1. Introduction
2. Results and Discussion
2.1. Examination of Particle Morphologies
2.2. Crystal Structure and Microstructure
2.3. Chemical Bonding Formations
2.4. Specific Surface Area and Particle Size of the WT Powders
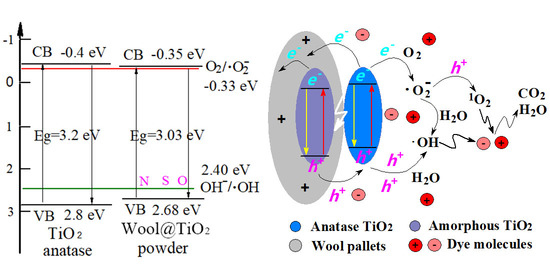
2.5. Band-Gap Structure
2.6. Separation Efficiency of Photogenerated Electron–Hole Pairs
2.7. Photocatalytic Performance
2.7.1. Optimization of Photocatalytic Degradation Efficiency
- (a)
- Optimization of the amount of TBT
- (b)
- Effect of the wavelength of light exposure
- (c)
- Effect of the irradiation intensity
- (d)
- Effect of the dosage of the WT photocatalysts
- (e)
- Effects of the initial dye concentrations
- (f)
- Effect of the pH value of the dye solutions
- (g)
- Effect of the oxidant agent, H2O2
- (h)
- Effects of air and N2
- (i)
- Comparison of the photodegradation efficiency of the WT powders onto the MB dye solutions
2.7.2. Photodegradation Effect of the WT Powders onto Other Dye Solutions
2.7.3. Reusability and Stability of the WT Powders
2.8. Identification of Reactive Radical Species
2.9. Photocatalytic Mechanism of the WT Powders
3. Experimental Section
3.1. Materials and Reagents
3.2. Fabrication of WT Hybrid Composite Powders
3.2.1. Degreasing of Wool Fibers
3.2.2. Fabrication of Wool Pallets
3.2.3. Swelling of Wool Pallets
3.2.4. Loading of TiO2 Nanoparticles on Wool Pallets
3.3. Characterization Methods
3.4. Measurements of Photocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nazari, A.; Montazer, M.; Dehghani-Zahedani, M. Simultaneous dyeing and mothproofing of wool against Dermestes Maculatus with madder optimized by statistical model. Clean. Technol. Environ. 2014, 16, 1675–1686. [Google Scholar] [CrossRef]
- Aksakal, B.; Koc, K.; Bozdogan, A.; Tsobkallo, K. Uniaxial tensile properties of TiO2 coated single wool fibers by sol-gel method: The effect of heat treatment. J. Appl. Polym. Sci. 2013, 130, 898–907. [Google Scholar] [CrossRef]
- Patil, K.; Rajkhowa, R.; Wang, X.; Lin, T. Review on fabrication and applications of ultrafine particles from animal protein fibres. Fibers Polym. 2014, 15, 187–194. [Google Scholar] [CrossRef]
- Patil, K.; Smith, S.V.; Rajkhowa, R.; Tsuzuki, T.; Wang, X.; Lin, T. Milled cashmere guard hair powders: Absorption properties to heavy metal ions. Powder Technol. 2012, 218, 162–168. [Google Scholar] [CrossRef]
- Atef El-Sayed, A.; Salama, M.; Kantouch, A.A.M. Wool micro powder as a metal ion exchanger for the removal of copper and zinc. Desalin. Water Treat. 2014, 56, 1010–1019. [Google Scholar] [CrossRef]
- Li, W.B.; Ke, G.Z.; Li, G.W.; Xu, W.L. Study on the structure and properties of viscose/wool powder blended fibre. Fibres Text. East. Eur. 2015, 23, 26–29. [Google Scholar]
- Wen, G.; Cookson, P.G.; Liu, X.; Wang, X.G. The effect of pH and temperature on the dye sorption of wool powders. J. Appl. Polym. Sci. 2010, 116, 2216–2226. [Google Scholar] [CrossRef]
- Naik, R.; Wen, G.Q.; Dharmaprakash, M.S.; Hureau, S.; Uedono, A.; Wang, X.G.; Liu, X.; Cookson, P.G.; Smith, S.V. Metal ion binding properties of novel wool powders. J. Appl. Polym. Sci. 2010, 115, 1642–1650. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.L.; Wang, X.G. Characterization of hot-pressed films from superfine wool powder. J. Appl. Polym. Sci. 2008, 108, 2852–2856. [Google Scholar] [CrossRef]
- Ke, G.Z.; Xu, W.L. Preparation and properties of superfine wool powder/chitosan complex membrane. J. Text. Inst. 2012, 103, 1183–1188. [Google Scholar] [CrossRef]
- Ke, G.Z.; Li, W.B. Preparation of viscose/wool powder blended fibre and optimization of its acid dyeing. Indian J. Fibres Text. 2016, 41, 195–199. [Google Scholar]
- Chang, H.B.; Li, Q.S.; Xu, C.J.; Li, R.M.; Wang, H.X.; Bu, Z.W.; Lin, T. Wool powder: An efficient additive to improve mechanical and thermal properties of poly (propylene carbonate). Compos. Sci. Technol. 2017, 153, 119–127. [Google Scholar] [CrossRef]
- Tang, B.; Zeng, T.; Liu, J.; Zhou, J.; Ye, Y.; Wang, X.G. Waste fiber powder functionalized with silver nanoprism for enhanced Raman scattering analysis. Nanoscale Res. Lett. 2017, 12, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.A.; Salama, M.; El-Rafie, M.H.; Emam, H.E. Modified rice straw as a template in syntheses of nano TiO2 loaded on wool fibers for wastewater treatment. J. Nat. Fibers 2017, 14, 297–309. [Google Scholar] [CrossRef]
- Li, H.Q.; Hu, J.T.; Wang, X.J.; An, L.H. Development of a bio-inspired photo-recyclable feather carbon adsorbent towards removal of amoxicillin residue in aqueous solutions. Chem. Eng. J. 2019, 373, 1380–1388. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Lian, J.H.; Ma, Q.; Bai, Y.; Tong, J.H.; Zhong, J.B.; Wang, R.M.; Huang, H.H.; Su, B.T. Photodegradation of Rhodamine B over a novel photocatalyst of feather keratin decorated CdS under visible light irradiation. New J. Chem. 2015, 39, 7112–7119. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, X.; Zeng, T.; Lin, X.; Zhou, J.; Ye, Y.; Wang, X.G. In situ synthesis of gold nanoparticles on wool powder and their catalytic application. Materials 2017, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.B.; Li, Q.S.; Cui, X.M.; Wang, H.X.; Bu, Z.W.; Qiao, C.Z.; Lin, T. Conversion of carbon dioxide into cyclic carbonates using wool powder-KI as catalyst. J. CO2 Util. 2018, 24, 174–179. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E. Self-cleaning and color reduction in wool fabric by nano titanium dioxide. J. Text. Inst. 2011, 102, 343–352. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoudd, W.A.; Leung, S.K. Understanding photocatalytic behavior on biomaterials: Insights from TiO2 concentration. J. Colloid Interface Sci. 2009, 339, 424–433. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.W.; Zhang, X.T.; Mao, N.T. Photocatalytic effects of wool fibers modified with solely TiO2 nanoparticles and N-doped TiO2 nanoparticles by using hydrothermal method. Chem. Eng. J. 2014, 254, 106–114. [Google Scholar] [CrossRef]
- Bozzi, A.; Yuranova, T.; Kiwi, J. Self-cleaning of wool-polyamide and polyester textiles by TiO2-rutile modification under daylight irradiation at ambient temperature. J. Photochem. Photobiol. A Chem. 2005, 172, 27–34. [Google Scholar] [CrossRef]
- Daoud, W.A.; Leung, S.K.; Tung, W.S.; Xin, J.H.; Cheuk, K.; Qi, K. Self-cleaning keratins. Chem. Mater. 2008, 20, 1242–1244. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Photocatalytic self-cleaning keratins: A feasibility study. Acta Biomater. 2009, 5, 50–56. [Google Scholar] [CrossRef]
- Zhang, H.; Millington, K.R.; Wang, X. The photostability of wool doped with photocatalytic titanium dioxide nanoparticles. Polym. Degrad. Stab. 2009, 94, 278–283. [Google Scholar] [CrossRef]
- Wang, L.Y.; Daoud, W.A. UV-induced photocatalytic cashmere fibers. Materials 2017, 12, 1414. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, D.; Han, Y.; Tang, Q.; Wu, H.L.; Mao, N.T. High photoactivity rutile-type TiO2 particles co-doped with multiple elements under visible light irradiation. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M.E.; Puca, M.; Bravo, J.P.; Bafico, J.; Orto, V.C.D.; Copello, G.J. Dual adsorbent-photocatalytic keratin-TiO2 nanocomposite for trimethoprim removal from wastewater. New J. Chem. 2020, 44, 10964–10972. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H. Modification of wool fabric treated with tetrabutyl titanate by hydrothermal method. J. Text. Inst. 2012, 103, 1108–1115. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.Y.; Mao, N.T. Functional modification with TiO2 nanoparticles and simultaneously dyeing of wool fibers in a one-pot hydrothermal process. Ind. Eng. Chem. Res. 2014, 53, 2030–2041. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, J.; Zhang, X.T. Hydrothermal treatment of wool fibers with tetrabutyl titanate and chitosan. J. Nat. Fibers 2015, 12, 518–530. [Google Scholar] [CrossRef]
- Zhang, J.H.; Fu, D.; Wang, S.Q.; Hao, R.L.; Xie, Y.X. Photocatalytic removal of chromium(VI) and sulfite using transition metal (Cu, Fe, Zn) doped TiO2 driven by visible light: Feasibility, mechanism and kinetics. J. Ind. Eng. Chem. 2019, 80, 23–32. [Google Scholar] [CrossRef]
- Xu, W.L.; Cui, W.G.; Li, W.B.; Guo, W.Q. Development and characterizations of super-fine wool powder. Powder Technol. 2004, 140, 136–140. [Google Scholar] [CrossRef]
- Kotlhao, K.; Mtunzi, F.M.; Pakade, V.; Ejidike, I.P.; Klink, M.J. Synthesis, characterization and evaluation of Ag-TiO2nanocomposites for photo-catalytic degradation of selected Chlorophenols. Dig. J. Nanomater. Biostruct. 2018, 13, 835–846. [Google Scholar]
- Behzadnia, A.; Montazer, M.; Rad, M.M. In situ photo sonosynthesis of organic/inorganic nanocomposites on wool fabric introducing multifunctional properties. Photochem. Photobiol. 2016, 92, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Fu, X.X.; Shi, L.; Yu, S.Q.; Qian, G.D.; Wang, Z.Y. Ultrasonic-induced nanocomposites with anatase@amorphous TiO2 core-shell structure and their photocatalytic activity. RSC Adv. 2016, 6, 67444–67448. [Google Scholar] [CrossRef]
- Calisir, M.D.; Gungor, M.; Ddmir, A.; Kilic, A.; Khan, M.M. Nitrogen-doped TiO2 fibers for visible-light-induced photocatalytic activities. Ceram. Int. 2020, 46, 16743–16753. [Google Scholar] [CrossRef]
- Bai, X.; Jia, J.; Du, Y.Y.; Hu, X.Y.; Li, J.L.; Liu, E.Z.; Fan, J. Multi-level trapped electrons system in enhancing photocatalytic activity of TiO2 nanosheets for simultaneous reduction of Cr (VI) and RhB degradation. Appl. Surf. Sci. 2020, 503, 144298. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, C.; Hong, J.M.; Chang, C.T. Preparation of Pt/TiO2/graphene/polyethylene sheets via a facile molding process for azo dye electrodegradation. J. Nanosci. Nanotechnol. 2020, 20, 3287–3294. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Zhou, Q.; Tsuzuki, T.; Morton, D.A.V.; Wang, X.G. Ultrafine wool powders and their bulk properties. Powder Technol. 2012, 224, 183–188. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.H.; Chen, Q.H.; Liu, G.C.; Zhou, Y.M.; Ma, D.; Cui, C.Y.; Ma, J.; Xin, Y.J. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Facile preparation of new nanohybrids for enhancing photocatalytic activity toward removal of organic dyes under visible light irradiation. J. Phys. Chem. Solids. 2020, 140, 109271. [Google Scholar] [CrossRef]
- Mutyala, S.; Sadiq, M.M.J.; Gurulakshmi, M.; Suresh, C.; Bhat, D.K.; Shanthi, K.; Mathiyarasu, J. Disintegration of flower-like MoS2 to limply allied layers on spherical nanoporous TiO2: Enhanced visible-light photocatalytic degradation of methylene blue. J. Nanosci. Nanotechnol. 2020, 20, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.J.; Han, D.X.; Niu, L. Ag supported Z-scheme WO2.9/g-C3N4 composite photocatalyst for photocatalytic degradation under visible light. Appl. Surf. Sci. 2020, 501, 144258. [Google Scholar] [CrossRef]
- Chai, Y.Y.; Ding, J.; Wang, L.; Liu, Q.Q.; Ren, J.; Dai, W.L. Enormous enhancement in photocatalytic performance of Ag3PO4/HAp composite: A Z-scheme mechanism insight. Appl. Catal. B Environ. 2015, 179, 29–36. [Google Scholar] [CrossRef]
- Paul, K.K.; Ghosh, R.; Giri, P.K. Mechanism of strong visible light photocatalysis by Ag2O-nanoparticle-decorated monoclinic TiO2 (B) porous nanorods. Nanotechnology 2016, 27, 315703. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Meng, X.D.; Jing, P.T.; Sun, M.Y.; Zhao, J.L.; Li, H.B. Enhancement of electron transfer from CdSe core/shell quantum dots to TiO2 films by thermal annealing. J. Lumin. 2011, 142, 196–201. [Google Scholar] [CrossRef]
- Chen, W.D.; Zhang, H.; Chen, T.Y.; Yang, L.M.; Wu, H.L.; Tong, Z.; Mao, N.T. TiO2 modified orthocortical and paracortical cells having enhanced photocatalytic degradation and photoreduction properties. Nanotechnology 2021, 32, 025714. [Google Scholar] [CrossRef]
- Ghosh, J.P.; Langford, C.H.; Achari, G. Characterization of an LED based photoreactor to degrade 4-chlorophenol in an aqueous medium using coumarin (C-343) sensitized TiO2. J. Phys. Chem. A 2008, 112, 10310–10314. [Google Scholar] [CrossRef]
- Yan, X.L.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Mitoraj, D.; Lamdab, U.; Kangwansupamonkon, W.; Pacia, M.; Macyk, W.; Wetchakun, N.; Beranek, R. Revisiting the problem of using methylene blue as a model pollutant in photocatalysis: The case of InVO4/BiVO4 composites. J. Photochem. Photobiol. A 2018, 366, 103–110. [Google Scholar] [CrossRef]
- Di, G.L.; Zhu, Z.L.; Dai, Q.; Zhang, H.; Shen, X.L.; Qiu, Y.L.; Huang, Y.Y.; Yu, J.N.; Yin, D.Q.; Kueppers, S. Wavelength-dependent effects of carbon quantum dots on the photocatalytic activity of g-C3N4 enabled by LEDs. Chem. Eng. J. 2020, 379, 122296. [Google Scholar] [CrossRef]
- Qiu, R.L.; Song, L.; Mo, Y.Q.; Zhang, D.D.; Brewer, E. Visible light induced photocatalytic degradation of phenol by polymer-modified semiconductors: Study of the influencing factors and the kinetics. React. Kinet. Catal. Lett. 2008, 94, 183–189. [Google Scholar] [CrossRef]
- Zhu, C.S.; Li, Y.L.; Yang, Y.W.; Chen, Y.X.; Yang, Z.G.; Wang, P.; Feng, W.L. Influence of operational parameters on photocatalytic decolorization of a cationic azo dye under visible-light in aqueous Ag3PO4. Inorg. Chem. Commun. 2020, 115, 107850. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Patrucco, A.; Caringella, R.; Montarsolo, A.; Zoccola, M.; Pozzo, P.D. Preparation of keratin-based microcapsules for encapsulation of hydrophilic molecules. Ultrason. Sonochem. 2018, 40, 527–532. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.A.P.; Araujo, B.A.; da Silva, K.S.; Silva, C.B.; de Lima, G.G.C.; Vieira, F.F.; de Medeiros, K.M. Advanced oxidative process by heterogeneous photocatalysis for chemical laboratories effluents treatment. Desalin. Water Treat. 2020, 174, 248–257. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Rahimi, S.; Ayati, B.; Rezaee, A. Kinetic modeling and determination role of sono/photo nanocatalyst-generated radical species on degradation of hydroquinone in aqueous solution. Environ. Sci. Pollut. R. 2016, 23, 12185–12198. [Google Scholar] [CrossRef]
- Pastrana-Martinez, L.M.; Pereira, N.; Lima, R.; Faria, J.L.; Gomes, H.T.; Silva, A.M.T. Degradation of diphenhydramine by photo-Fenton using magnetically recoverable iron oxide nanoparticles as catalyst. Chem. Eng. J. 2015, 261, 45–52. [Google Scholar] [CrossRef]
- Koh, P.W.; Hatta, M.H.M.; Ong, S.T.; Yuliati, L.; Lee, S.L. Photocatalytic degradation of photosensitizing and non-photosensitizing dyes over chromium doped titania photocatalysts under visible light. J. Photochem. Photobiol. A 2017, 332, 215–223. [Google Scholar] [CrossRef]
- Cestellos-Blanco, S.; Zhang, H.; Kim, J.M.; Shen, Y.X.; Yang, P.D. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat. Catal. 2020, 3, 245–255. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.X.; Zhang, M.; Yang, J.J.; Zhang, Z.J. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Alivov, Y.; Grant, T.; Capan, C.; Iwamoto, W.; Pagliuso, P.G.; Molloi, S. Origin of magnetism in undoped TiO2 nanotubes. Nanotechnology 2013, 24, 275704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Liang, Y.Q.; Huang, H.L.; Liu, X.Y.; Li, Q.; Chen, L.X.; Xu, D.S. Stable and highly efficient photocatalysis with lead-free double-perovskite of Cs2AgBiBr6. Angew. Chem. Int. Ed. 2019, 58, 7263–7267. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.C.; Cong, Y.Q.; Zhang, Y. Photo-sensitized degradation of dye pollutants on amorphous TiO2 under visible light irradiation. Chin. J. Catal. 2011, 32, 1076–1082. [Google Scholar] [CrossRef]
- Krylova, G.; Na, C.Z. Photoinduced crystallization and activation of amorphous titanium dioxide. J. Phys. Chem. C 2015, 119, 12400–12407. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.Y.; Jin, F.L.; Lee, S.Y.; Choi, H.S.; Park, S.J. Combined effect of corona discharge and enzymatic treatment on the mechanical and surface properties of wool. J. Ind. Eng. Chem. 2014, 20, 179–183. [Google Scholar] [CrossRef]
- Frunza, L.; Diamandescu, L.; Zgura, I.; Frunza, S.; Ganea, C.P.; Negrila, C.C.; Enculescu, M.; Birzu, M. Photocatalytic activity of wool fabrics deposited at low temperature with ZnO or TiO2 nanoparticles: Methylene blue degradation as a test reaction. Catal. Today 2018, 306, 251–259. [Google Scholar] [CrossRef]
- Liu, H.J.; Chen, P.; Yuan, X.Y.; Zhang, Y.X.; Huang, H.W.; Wang, L.A.; Dong, F. Pivotal roles of artificial oxygen vacancies in enhancing photocatalytic activity and selectivity on Bi2O2CO3 nanosheets. Chin. J. Catal. 2019, 40, 620–630. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.Y.; Lin, H.L.; Luo, B.D.; Chen, S.F. Chemical etching preparation of BiOI/BiOBr heterostructures with enhanced photocatalytic properties for organic dye removal. Chem. Eng. J. 2012, 185, 91–99. [Google Scholar] [CrossRef]
- Eser, F.; Onal, A. Dyeing of wool and cotton with extract of the nettle (Urtica dioica L.) leaves. J. Nat. Fibers 2015, 12, 222–231. [Google Scholar] [CrossRef]
- Tian, B.; Chen, F.; Tong, Y.X.; Li, L.; Zheng, Y.F.; Liu, Y.; Li, Q.Z. Phase transition of Ni-Mn-Ga alloy powders prepared by vibration ball milling. J. Alloys Compd. 2011, 509, 4563–4568. [Google Scholar] [CrossRef]
- Shirgholami, M.A.; Karimi, L.; Mirjalili, M. Multifunctional modification of wool fabric using graphene/TiO2 nanocomposite. Fibers Polym. 2016, 17, 220–228. [Google Scholar] [CrossRef]
- Goswami, S.; Sharma, A.K. Investigation of the optical behavior of indium oxide thin films with the aid of spectroscopic ellipsometry technique. Appl. Surf. Sci. 2019, 495, 143609. [Google Scholar] [CrossRef]
- Taguchi, S.; Saruyama, M.; Teranishi, T.; Kanemitsu, Y. Quantized auger recombination of biexcitons in CdSe nanorods studied by time-resolved photoluminescence and transient-absorption spectroscopy. Phys. Rev. B 2011, 83, 155324. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.Z.; Li, X.W.; Jiang, J.; Wang, K.; Zhang, H.Q.; Yu, A.M.; Zhang, Z.W. Application of Cu2+-based electron spin resonance spectroscopy in measurement of antioxidant capacity of fruits. Anal. Bioanal. Chem. 2019, 411, 6677–6686. [Google Scholar] [CrossRef]
- Ghayeb, Y.; Momeni, M.M. Novel visible-light-responsive photo-catalysts based on palladium decorated nanotube films fabricated on titanium substrates. Ceram. Int. 2016, 42, 11209–11216. [Google Scholar] [CrossRef]
- O’Dell, J.W. Method 410.4-The Determination of chemical oxygen demand by semi-automated colorimetry. In Methods for the Determination of Metals in Environmental Samples, 1st ed.; Elsevier: Cincinnati, OH, USA, 1996; pp. 509–521. [Google Scholar]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LR under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Zhang, H.; Zhang, X.; Guo, Y.; Yang, L.; Wu, H.; Mao, N. Photocatalytic Properties of Core-Shell Structured Wool-TiO2 Hybrid Composite Powders. Catalysts 2021, 11, 12. https://doi.org/10.3390/catal11010012
Gu H, Zhang H, Zhang X, Guo Y, Yang L, Wu H, Mao N. Photocatalytic Properties of Core-Shell Structured Wool-TiO2 Hybrid Composite Powders. Catalysts. 2021; 11(1):12. https://doi.org/10.3390/catal11010012
Chicago/Turabian StyleGu, Haoshuai, Hui Zhang, Xinyue Zhang, Yani Guo, Limeng Yang, Hailiang Wu, and Ningtao Mao. 2021. "Photocatalytic Properties of Core-Shell Structured Wool-TiO2 Hybrid Composite Powders" Catalysts 11, no. 1: 12. https://doi.org/10.3390/catal11010012
APA StyleGu, H., Zhang, H., Zhang, X., Guo, Y., Yang, L., Wu, H., & Mao, N. (2021). Photocatalytic Properties of Core-Shell Structured Wool-TiO2 Hybrid Composite Powders. Catalysts, 11(1), 12. https://doi.org/10.3390/catal11010012