Atomically Dispersed Catalytic Sites: A New Frontier for Cocatalyst/Photocatalyst Composites toward Sustainable Fuel and Chemical Production
Abstract
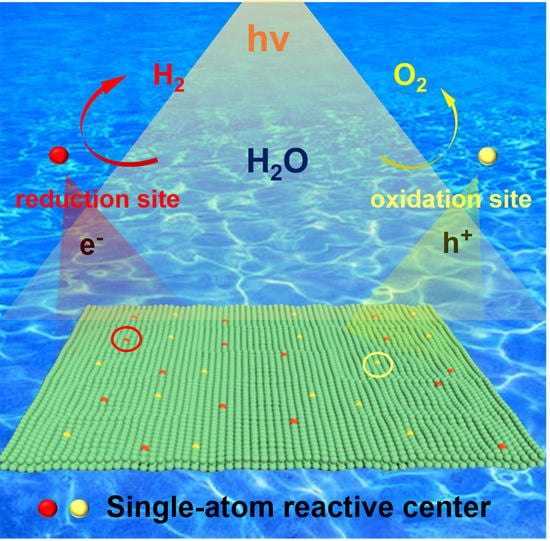
:1. Introduction
2. Structure of SAPCs
2.1. Support Materials
2.1.1. Inorganic Materials
2.1.2. Organic Materials


2.1.3. Carbon-Based Materials
2.1.4. Metals
2.1.5. Composites
2.2. Single-Atom Cocatalysts
2.2.1. Noble Metals
2.2.2. Non-Noble Metals
3. Synthesis of SAPCs
3.1. Impregnation Method
3.2. Co-Precipitation Method
3.3. Photo-Deposition Method
3.4. Atomic Layer Deposition Method
3.5. Other Methods

4. Applications of SAPCs for Photocatalytic Water Splitting
| Supports | Single Atoms | Coordination | Loaded Content | Synthesis Methods | Products | Production Rate | AQE | |
|---|---|---|---|---|---|---|---|---|
| 1 [27] | TiO2 nanosheet | Rh | Rh-O | 2.6 at.% | co-precipitation | H2 | 51 umol h−1 | / |
| 2 [98] | g-C3N4 | Pt | Pt-N | 0.16 wt.% | impregnation | H2 | 318 umol h−1 | / |
| 3 [54] | g-C3N4 | Pt2+ | Pt-N | 0.18 wt.% | co-precipitation | H2 | 605 umol g−1 h−1 | / |
| 4 [113] | g-C3N4 | Co | Co1-N4 | 1 wt.% | ALD | H2 | 10.8 umol h−1 | 10.3%, 420 nm |
| 5 [127] | Ag cluster | Pt | Pt-Ag | 4 at.% | co-precipitation | H2 | 39.7 umol h−1 | / |
| 6 [56] | P doped g-C3N4 | Co | Co1-P4 | 0.40.% | impregnation | H2. O2 | 126.8 umol g−1 h−1 | 0.16%, AM 1.5 illumination |
| 7 [96] | g-C3N4 | Pt | Pt-N (maybe) | / | impregnation | H2 | 12.7 umol g−1, 4h | / |
| 8 [22] | TiO2 | Pt | Pt-O | 0.6 wt.% | photo-deposition | H2 | 85.4 umol h−1 | / |
| 9 [76] | NG | Co | / | 0.25 wt.% (Co-NG) | impregnation | H2 | 1382 umol h−1 | 50.5%, 420 nm |
| 10 [104] | g-C3N4 | Pd | surface:Pt-N;interlayer: Pt-N, Pt-C | 0.33 wt.% | photo-deposition | H2 | 6688 umol g−1 h−1 | 4%, 420 nm |
| 11 [137] | g-C3N4 | Pt | Pt1-N4 | 0.11 wt.% | photo-deposition | H2 | 42.1 umol h−1 | / |
| 12 [95] | Al- porphyrinic MOF | Pt | Pt-N | 0.29 wt.% | impregnation | H2 | 129 umol g−1 h−1 | / |
| 13 [121] | Zr–porphyrinic MOF | Ir, Pt, Ru, Au, Pd | Pt-N4Cl2 | 1.41 (Ir), 2.74 (Pt), 1.92 (Ru), 1.18 (Au), 3.68 (Pd), wt.% | impregnation | H2 | 201.9 umol g−1 h−1 | / |
| 14 [138] | g-C3N4 | PtⅡ | Pt-N | / | impregnation | H2 | 140 umol g−1 h−1 | 1.5%, 420 nm |
| 15 [35] | mesoporous TiO2 | Cu | Cu-O2.5 | <0.3 wt.% | impregnation | H2 | / | / |
| 16 [38] | CdS nanowires | Pt | Pt-S4 | 0.27–0.98 wt.% | impregnation | H2 | 47.41 mmol g−1 h−1 | / |
| 17 [140] | g-C3N4 | Pt | Pt-C/Pt-N | 0.17–1.7% | impregnation | H2 | 34.2 mmol h−1 | / |
| 18 [111] | MOF | Co | Co-N2Cl2O | 3.3 wt.% | co-precipitation | H2 | 27.853 mmol g−1, 40h | / |
| 19 [141] | NG | Co | Co-N2C | 3.5 wt.% (Co-NG) | impregnation | H2 | 677.44 umol g−1 h−1 | / |
| 20 [122] | NG | Ni | Ni-N | 0.26 wt.% | impregnation | H2 | 1351.1 umol h−1 | 48.2%, 420 nm |
| 21 [100] | TiO2 | (CH3)2Pt(COD) | O-Pt-O | / | impregnation | H2 | / | 12%, 225–387 nm |
| 22 [114] | TiO2 | Cu, Co, Fe, Ni, Rh | Cu-O | 0.75 wt.% | impregnation | H2 | 16.6 mmol g−1 h−1 | 45.5%, 340 nm |
| 23 [42] | GaS nanosheet | Ru(IV) | Ru1-S6 | 2.4 wt.% | impregnation | H2, O2 | 340 umol g−1 h−1 | / |
| 24 [97] | MOF−808-EDTA | Pt | Pt-N2O2 | 0.98 wt.% | impregnation | H2 | 68.33 mmol g−1 h−1 | 67.6%, 420 nm |
| 25 [101] | g-C3N4 | Pd | Pd-N | 0.1 wt.% | impregnation | H2 | 728 umol g−1 h−1 | / |
| 26 [83] | CdS@CDs | Pt | Pt-S | 1.15% | impregnation | H2 | 45.5 mmol g−1 h−1 | 29.8%, 400 nm |
| 27 [142] | zeolitic imidazole framework | Co | / | 0.57% | co-precipitation | H2 | 6420 umol g−1 h−1 | / |
| 28 [107] | g-C3N4 | Pt, Au | Pt-N, Au-N | 0.6 Pt, 0.8 (Au), wt.% | impregnation | H2, O2 | H2: 285 umol g−1 h−1, H2:O2 = 2:1 | / |
| 29 [77] | N doped CDs | Co | Co-N4 | 3.27 wt.% | co-precipitation | O2 | 245 umol g−1, 4h | / |
| 30 [55] | g-C3N4 | Au | Au-O | 0.18 wt.% | impregnation | H2 | 789.1 nmol h−1 | / |
| 31 [117] | g-C3N4 | Fe | Pt-N (DFT) | 0.5 at.% | impregnation | H2 | 3390 umol g−1 h−1 | 6.89%, 420 nm |
| 32 [129] | g-C3N4 | Pt | Pt-N/O/Cl | 0.35 mg m−2 | photo-deposition | H2 | 174.5 mmol g−1 h−1 | 0.544%, 420 nm |
| 33 [66] | MOF | Pt | Pt-N | 12 wt.% | co-precipitation | H2 | 11320 umol g−1 h−1 | / |
| 34 [143] | TiO2 | Pt | Pt-O | 0.36 wt.% | photo-deposition | H2 | 1077 umol h−1 | 21.7%, 365 nm |
| 35 [94] | defective TiO2 | Pt | Pt-O | 0.02wt% | impregnation | H2 | 4458 umol g−1 h−1 | / |
| 36 [103] | g-C3N4 | Rh | Rh-P | / | impregnation | H2 | 166.28 umol, 4h | / |
| 37 [105] | g-C3N4 | Co | Co-P | 0.13 wt.% | impregnation | O2, H2O2 | O2: 15.5 umol, 4h; | / |
| 38 [88] | TiO2 | Pt | / | 0.03–0.47 at.%. | impregnation | H2 | / | / |
| 39 [144] | g-C3N4 | Ag | Ag-C2N2 | 3.7 wt.% | co-precipitation | H2 | 1.8 mmol g−1 h−1 | / |
| 40 [116] | g-C3N4 | Ni | Ni-C/N | 0.5 at% | impregnation | H2 | 354.9 umol g−1 h−1 | / |
| 41 [74] | N-doped CDs | Pt | Pt-C | 0.2 wt.% | photo-deposition | H2 | 175.3 umol h−1 cm−1 | / |
| 42 [124] | Zn0.25Cd0.75S QDs | Ni | Ni-S | 0.15-0.125 at% | co-precipitation | H2 | 18.87 mmol g−1 h−1 | / |
| 43 [123] | coordination polymers | Cu | Cu-N | / | co-precipitation | H2 | 57.64 mmol g−1 h−1 | / |
| 44 [136] | g-C3N4 | Pt | Pt-N | 8.7 wt.% | ion exchange | H2 | 22650 umol g−1 h−1 | 22.5%, 420 nm |
| 45 [39] | CdS | Ni | Ni-O | 2.85 wt.% | photo-deposition | H2 | 630.1 mmol g−1 h−1 | / |
| 46 [145] | TiO2 spheres | Ru | Ru-O | 0.93 wt.% | impregnation | H2 | 7.2 mmol g−1 h−1 | / |
| 47 [99] | g-C3N4 | Pt | Pt-N | / | impregnation | H2 | 14.7 mmol g−1 h−1 | 38.8%,435 nm |
| 48 [106] | CdS | Pt | Pt-S | 0.50 wt.% | impregnation | H2 | 24.17 mmol g−1 h−1 | 46%, 420 nm |
| 49 [146] | TiO2 nanosheet | Co | Co-O | 1.11 wt.% | impregnation | H2 | 1.682 mmol g−1 h−1 | / |
| 50 [93] | g-C3N4 | Pt | Pt-C4 | 1 wt.% | Photo-deposition | H2 | 25.4 umol h−1 | 0.5%, AM 1.5 illumination |
| 51 [45] | Cs2SnI6 | Pt | Pt-I3 | 0.12 wt.% | impregnation | H2 | 430 umol g−1 h−1 | / |
| 52 [147] | unimolecular micelles | Pt | Pt-N, Pt-C-N | 4.1 wt.% | co-precipitation | H2 | 49465 umol gPt−1 h−1 | / |
5. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fu, C.F.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, e1802106. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Wachs, I.E.; Phivilay, S.P.; Roberts, C.A. Reporting of Reactivity for Heterogeneous Photocatalysis. ACS Catal. 2013, 3, 2606–2611. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Mitchell, S.; Perez-Ramirez, J. Single Atom Catalysis: A Decade of Stunning Progress and the Promise for a Bright Future. Nat. Commun. 2020, 11, 4302. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, K.-X.; Guo, X.-X.; Chen, J.-S. Single-Site Photocatalysts with a Porous Structure. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2099–2112. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, H.; Shen, S. Single Metal Atom Photocatalysis. Small Methods 2019, 3, 1800447. [Google Scholar] [CrossRef]
- Zhu, Y.; Sokolowski, J.; Song, X.; He, Y.; Mei, Y.; Wu, G. Engineering Local Coordination Environments of Atomically Dispersed and Heteroatom-Coordinated Single Metal Site Electrocatalysts for Clean Energy-Conversion. Adv. Energy Mater. 2019, 10, 1902844. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Chen, Y.; Fu, W.-F.; Lv, X.-J. Single-Atom Catalysts for Photocatalytic Reactions. ACS Sustain. Chem. Eng. 2019, 7, 6430–6443. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, B.; Ran, J.; Davey, K.; Qiao, S.Z. Atomic-Level Reactive Sites for Semiconductor-Based Photocatalytic CO2 Reduction. Adv. Energy Mater. 2020, 10, 1903879. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Recent Progress in Single-Atom Catalysts for Photocatalytic Water Splitting. Sol. RRL 2020, 4, 2000283. [Google Scholar] [CrossRef]
- Zeng, L.; Xue, C. Single Metal Atom Decorated Photocatalysts: Progress and Challenges. Nano Res. 2020, 14, 934–944. [Google Scholar] [CrossRef]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Ji, S.; Qu, Y.; Wang, T.; Chen, Y.; Wang, G.; Li, X.; Dong, J.; Chen, Q.; Zhang, W.; Zhang, Z.; et al. Rare-Earth Single Erbium Atoms for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. Engl. 2020, 59, 10651–10657. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, S.; Li, T.; Liu, Q.; Jiang, T.; Guo, Y.; Luo, J.-L. Atomically Dispersed Pt on Specific TiO2 Facets for Photocatalytic H2 Evolution. J. Catal. 2017, 353, 250–255. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Doping of WO3 for Photocatalytic Water Splitting: Hints from Density Functional Theory. J. Phys. Chem. C 2012, 116, 8901–8909. [Google Scholar] [CrossRef]
- Wang, J.; Xia, T.; Wang, L.; Zheng, X.; Qi, Z.; Gao, C.; Zhu, J.; Li, Z.; Xu, H.; Xiong, Y. Enabling Visible-Light-Driven Selective CO2 Reduction by Doping Quantum Dots: Trapping Electrons and Suppressing H2 Evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 16447–16451. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic Water Splitting with a Quantum Efficiency of Almost Unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Dong, S.; Li, B.; Cui, X.; Tan, S.; Wang, B. Photoresponses of Supported Au Single Atoms on TiO2(110) through the Metal-Induced Gap States. J. Phys. Chem. Lett. 2019, 10, 4683–4691. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Pratsinis, S.E. Single Pd Atoms on TiO2 Dominate Photocatalytic NOx Removal. Appl. Catal. B 2018, 226, 127–134. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Y.; Wei, W.; Ma, X.; Li, M.; Huang, B. Effects of Single Metal Atom (Pt, Pd, Rh and Ru) Adsorption on the Photocatalytic Properties of Anatase TiO2. Appl. Surf. Sci. 2017, 426, 639–646. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 2002, 95, 735–758. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, S.; You, M.; Hong, S.; Wu, T.-S.; Soo, Y.-L.; Zhao, Z.; Jiang, G.; Jieshan, Q.; et al. Photocatalytic Fixation of Nitrogen to Ammonia by Single Ru Atom Decorated TiO2 Nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 6813–6820. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, H.; Zheng, H.; Zhang, P. Atomically Pt Implanted Nanoporous TiO2 Film for Photocatalytic Degradation of Trace Organic Pollutants in Water. Chem. Eng. J. 2020, 385, 123832. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, H.; Zhang, P. Isolated Pt Single Atomic Sites Anchored on Nanoporous TiO2 Film for Highly Efficient Photocatalytic Degradation of Low Concentration Toluene. J. Hazard. Mater. 2020, 388, 121746. [Google Scholar] [CrossRef] [PubMed]
- Trofimovaite, R.; Parlett, C.M.A.; Kumar, S.; Frattini, L.; Isaacs, M.A.; Wilson, K.; Olivi, L.; Coulson, B.; Debgupta, J.; Douthwaite, R.E.; et al. Single Atom Cu(I) Promoted Mesoporous Titanias for Photocatalytic Methyl Orange Depollution and H2 Production. Appl. Catal. B 2018, 232, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Luttrell, T.; Batzill, M. A Two-Dimensional Phase of TiO(2) with a Reduced Bandgap. Nat. Chem. 2011, 3, 296–300. [Google Scholar] [CrossRef]
- Ida, S.; Kim, N.; Ertekin, E.; Takenaka, S.; Ishihara, T. Photocatalytic Reaction Centers in Two-Dimensional Titanium Oxide Crystals. J. Am. Chem. Soc. 2015, 137, 239–244. [Google Scholar] [CrossRef]
- Nebel, R.; Macounová, K.M.; Tarábková, H.; Kavan, L.; Krtil, P. Selectivity of Photoelectrochemical Water Splitting on TiO2 Anatase Single Crystals. J. Phys. Chem. C 2019, 123, 10857–10867. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Q.; Chao, Y.; Wang, L.; Li, Y.; Chen, H.; Gu, L.; Guo, S. Partially Reduced Pd Single Atoms on CdS Nanorods Enable Photocatalytic Reforming of Ethanol into High Value-Added Multicarbon Compound. Chem 2021, 7, 1033–1049. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Li, G.; Duan, H.; Cheng, W.; Wang, C.; Hu, W.; Sun, Z.; Tan, H.; Li, N.; Ji, Q.; Wang, Y.; et al. Interlayer Photoelectron Transfer Boosted by Bridged Ru(IV) Atoms in GaS Nanosheets for Efficient Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 45561–45567. [Google Scholar] [CrossRef]
- Gupta, S.S.; van Huis, M.A. Intermetallic Differences at CdS-Metal (Ni, Pd, Pt, and Au) Interfaces: From Single-Atom to Subnanometer Metal Clusters. J. Phys. Chem. C Nanomater Interfaces 2019, 123, 9298–9310. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhang, H.; Dong, J.; Qiu, M.; Kong, J.; Zhang, Y.; Li, Y.; Xu, G.; Zhang, J.; Ye, J. Surface Step Decoration of Isolated Atom as Electron Pumping: Atomic-Level Insights into Visible-Light Hydrogen Evolution. Nano Energy 2018, 45, 109–117. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.; Zhao, S.; Wang, G.; Jiang, P.; Zhong, J.; Zhu, Y. Photochemical Preparation of Atomically Dispersed Nickel on Cadmium Sulfide for Superior Photocatalytic Hydogen Evolution. Appl. Catal. B 2020, 261, 118233. [Google Scholar] [CrossRef]
- Shoji, S.; Peng, X.; Yamaguchi, A.; Watanabe, R.; Fukuhara, C.; Cho, Y.; Yamamoto, T.; Matsumura, S.; Yu, M.-W.; Ishii, S.; et al. Photocatalytic Uphill Conversion of Natural Gas Beyond the Limitation of Thermal Reaction Systems. Nat. Catal. 2020, 3, 148–153. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, H.; Chao, Y.; Zhang, Q.; Zhang, W.; Lv, F.; Gu, L.; Zhao, Q.; Wang, N.; Wang, J.; et al. Single-Atom Pt-I3 Sites on All-Inorganic Cs2SnI6 Perovskite for Efficient Photocatalytic Hydrogen Production. Nat. Commun. 2021, 12, 4412. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.Z.; Chen, S.; Duan, M.; Xiong, J.; Zhu, C.; Long, R.; Hao, W.; Chi, Z.; et al. Isolated Single Atom Cobalt in Bi3O4Br Atomic Layers to Trigger Efficient CO2 Photoreduction. Nat. Commun. 2019, 10, 2840. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on g-C3N4 -Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water Under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Chen, Z.; Mitchell, S.; Vorobyeva, E.; Leary, R.K.; Hauert, R.; Furnival, T.; Ramasse, Q.M.; Thomas, J.M.; Midgley, P.A.; Dontsova, D.; et al. Stabilization of Single Metal Atoms on Graphitic Carbon Nitride. Adv. Funct. Mater. 2017, 27, 1605785. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kong, T.; Shen, S. Artificial Photosynthesis with Polymeric Carbon Nitride: When Meeting Metal Nanoparticles, Single Atoms, and Molecular Complexes. Small 2019, 15, e1900772. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, Z.; Xia, T.; Ju, H.; Zhang, K.; Long, R.; Xu, Q.; Wang, C.; Song, L.; Zhu, J.; et al. Implementing Metal-to-Ligand Charge Transfer in Organic Semiconductor for Improved Visible-Near-Infrared Photocatalysis. Adv. Mater. 2016, 28, 6959–6965. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Dai, C.; Liu, B.; Xue, C. Oxygen-Assisted Stabilization of Single-Atom Au During Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 24217–24221. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Cheng, W.; Cao, Y.; Liu, X.; Zhang, W.; Mou, X.; Jin, L.; Zheng, X.; Che, W.; et al. Single-Site Active Cobalt-Based Photocatalyst with a Long Carrier Lifetime for Spontaneous Overall Water Splitting. Angew. Chem. Int. Ed. Engl. 2017, 56, 9312–9317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, F.; Chen, J.; Fan, J.; Xiang, Q. Single Au Atoms Anchored on Amino-Group-Enriched Graphitic Carbon Nitride for Photocatalytic CO2 Reduction. ChemSusChem 2020, 13, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [PubMed]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and Photocatalysis by Metal Organic Frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; de Krafft, K.E.; Lin, W. Doping Metal-Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.-Z.; Li, X.; Wei, Z.; Xu, Y.-W.; Zhang, L.; Su, C.-Y. A Porous Rhodium(III)-Porphyrin Metal-Organic Framework as an Efficient and Selective Photocatalyst for CO2 Reduction. Appl. Catal. B 2018, 231, 173–181. [Google Scholar] [CrossRef]
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of Adsorption and Photosensitivity Capabilities into a Cationic Multivariate Metal-Organic Framework for Enhanced Visible-Light Photoreduction Reaction. Appl. Catal. B 2019, 253, 323–330. [Google Scholar] [CrossRef]
- Liu, M.; Mu, Y.-F.; Yao, S.; Guo, S.; Guo, X.-W.; Zhang, Z.-M.; Lu, T.-B. Photosensitizing Single-Site Metal−organic Framework Enabling Visible-Light-Driven CO2 Reduction for Syngas Production. Appl. Catal. B 2019, 245, 496–501. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal-Organic-Framework-Based Single-Atom Catalysts for Energy Applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal-Organic Framework. Angew. Chem. Int. Ed. Engl. 2016, 55, 14310–14314. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, T.; Chen, C.; Ji, Y.; Gong, X.; Mai, Y.; Zhou, Y. Ultrathin Metal-Organic Framework Nanosheets with Ultrahigh Loading of Single Pt Atoms for Efficient Visible-Light-Driven Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. Engl. 2019, 58, 10198–10203. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Tang, Y.; Huang, H.; Cui, H.; Mei, D.; Zhong, C. Single-Atom Pt–N3 Sites on the Stable Covalent Triazine Framework Nanosheets for Photocatalytic N2 Fixation. ACS Catal. 2020, 10, 2431–2442. [Google Scholar] [CrossRef]
- Zhong, W.; Sa, R.; Li, L.; He, Y.; Li, L.; Bi, J.; Zhuang, Z.; Yu, Y.; Zou, Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2019, 141, 7615–7621. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-Based Semiconductor Photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, L.; Zhao, Y.; Shi, R.; Waterhouse, G.I.N.; Huang, Y.C.; Zheng, L.; Zhang, T. Pd Single-Atom Catalysts on Nitrogen-Doped Graphene for the Highly Selective Photothermal Hydrogenation of Acetylene to Ethylene. Adv. Mater. 2019, 31, e1900509. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Y.; Dimitrov, S.D.; Steier, L.; Guo, S.; Li, X.; Feng, J.; Xie, F.; Fang, Y.; Sapelkin, A.; et al. Pt Single-Atoms Supported on Nitrogen-Doped Carbon Dots for Highly Efficient Photocatalytic Hydrogen Generation. J. Mater. Chem. A 2020, 8, 14690–14696. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Wang, Y.; Wang, J.; Zheng, X.; Zhu, J.; Song, L.; Zhang, W.; Xiong, Y. Heterogeneous Single-Atom Catalyst for Visible-Light-Driven High-Turnover CO2 Reduction: The Role of Electron Transfer. Adv. Mater. 2018, 30, e1704624. [Google Scholar] [CrossRef]
- Zhao, Q.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Effective and Durable Co Single Atomic Cocatalysts for Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 42734–42741. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Tu, X.; Liu, H.; Shu, M.; Si, R.; Ferguson, C.T.J.; Zhang, K.A.I.; Li, R. Single Atomically Anchored Cobalt on Carbon Quantum Dots as Efficient Photocatalysts for Visible Light-Promoted Oxidation Reactions. Chem. Mater. 2019, 32, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Luo, Q.; Liu, W.; Lin, Y.; Guan, Q.; Zheng, X.; Pan, H.; Zhu, J.; Sun, Z.; Wei, S.; et al. Quasi Pd1Ni Single-Atom Surface Alloy Catalyst Enables Hydrogenation of Nitriles to Secondary Amines. Nat. Commun. 2019, 10, 4998. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-Stephanopoulos, M.; Stamatakis, M.; Sykes, E.C.H. Pt/Cu Single-Atom Alloys as Coke-Resistant Catalysts for Efficient C-H Activation. Nat. Chem. 2018, 10, 325–332. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon Resonant Enhancement of Photocatalytic Water Splitting under Visible Illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-Driven Methane Dry Reforming with Single Atomic Site Antenna-Reactor Plasmonic Photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Qiu, S.; Shen, Y.; Wei, G.; Yao, S.; Xi, W.; Shu, M.; Si, R.; Zhang, M.; Zhu, J.; An, C. Carbon Dots Decorated Ultrathin CdS Nanosheets Enabling In-Situ Anchored Pt Single Atoms: A Highly Efficient Solar-Driven Photocatalyst for Hydrogen Evolution. Appl. Catal. B 2019, 259, 118036. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, R. Photochemical Deposition of Pt on CdS for H2 Evolution from Water: Markedly Enhanced Activity by Controlling Pt Reduction Environment. J. Phys. Chem. C 2013, 117, 783–790. [Google Scholar] [CrossRef]
- Tong, T.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Mechanistic Insight into the Enhanced Photocatalytic Activity of Single-Atom Pt, Pd or Au-Embedded g-C3N4. Appl. Surf. Sci. 2018, 433, 1175–1183. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-Abundant Cocatalysts for Semiconductor-Based Photocatalytic Water Splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, T.; Xu, T.; Li, Y.; Wang, C. Size Effect of Pt Co-Catalyst on Photocatalytic Efficiency of g-C3N4 for Hydrogen Evolution. Appl. Surf. Sci. 2019, 464, 36–42. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Osuagwu, B.; Zoppellaro, G.; Andryskova, P.; Tomanec, O.; Kment, S.; Zboril, R.; Schmuki, P. On the Controlled Loading of Single Platinum Atoms as a Co-Catalyst on TiO2 Anatase for Optimized Photocatalytic H2 Generation. Adv. Mater. 2020, 32, e1908505. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.; Fang, M.; Hsu, Y. Modulation of Interfacial Charge Dynamics of Semiconductor Heterostructures for Advanced Photocatalytic Applications. Coord. Chem. Rev. 2021, 438, 213876. [Google Scholar] [CrossRef]
- Lai, T.-H.; Katsumata, K.-I.; Hsu, Y.-J. In Situ Charge Carrier Dynamics of Semiconductor Nanostructures for Advanced Photoelectrochemical and Photocatalytic Applications. Nanophotonics 2020, 10, 777–795. [Google Scholar] [CrossRef]
- Chen, F.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Atomic-Level Charge Separation Strategies in Semiconductor-Based Photocatalysts. Adv Mater. 2021, 33, e2005256. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Ji, Y.; Batmunkh, M.; An, P.; Zhang, J.; Fu, Y.; Jia, B.; Li, Y.; Liu, S.; Ye, J.; et al. Breaking Platinum Nanoparticles to Single-Atomic Pt-C4 Co-catalysts for Enhanced Solar-to-Hydrogen Conversion. Angew. Chem. Int. Ed. Engl. 2021, 60, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2020, 59, 1295–1301. [Google Scholar] [CrossRef]
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H.L. Single Pt Atoms Confined into a Metal-Organic Framework for Efficient Photocatalysis. Adv. Mater. 2018, 30, 1705112. [Google Scholar] [CrossRef]
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Wang, Y. Single Pt Atoms Deposition on g-C3N4 Nanosheets for Photocatalytic H2 Evolution or NO Oxidation under Visible Light. Int. J. Hydrog. Energy 2017, 42, 27043–27054. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Liu, P.; Song, X.; Mei, D.; Tang, Y.; Wang, X.; Zhong, C. Metal-Organic Framework Encapsulated Single-Atom Pt Catalysts for Efficient Photocatalytic Hydrogen Evolution. J. Catal. 2019, 375, 351–360. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 6224–6229. [Google Scholar] [CrossRef]
- Jeantelot, G.; Qureshi, M.; Harb, M.; Ould-Chikh, S.; Anjum, D.H.; Abou-Hamad, E.; Aguilar-Tapia, A.; Hazemann, J.L.; Takanabe, K.; Basset, J.M. TiO2-Supported Pt Single Atoms by Surface Organometallic Chemistry for Photocatalytic Hydrogen Evolution. Phys. Chem. Chem. Phys. 2019, 21, 24429–24440. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, X.; Wang, L.; Xu, X.; Gan, L.; Si, Z.; Li, J.; Zhang, Q.; Liu, Y.; Zhao, Y.; et al. Atomic Palladium on Graphitic Carbon Nitride as a Hydrogen Evolution Catalyst under Visible Light Irradiation. Comm. Chem. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Nie, G.; Li, P.; Liang, J.-X.; Zhu, C. Theoretical Investigation on the Photocatalytic Activity of the Au/g-C3N4 Monolayer. J. Theor. Comput. Chem. 2017, 16, 1750013. [Google Scholar] [CrossRef]
- Chen, Z.; Bu, Y.; Wang, L.; Wang, X.; Ao, J.-P. Single-Sites Rh-Phosphide Modified Carbon Nitride Photocatalyst for Boosting Hydrogen Evolution under Visible Light. Appl. Catal. B 2020, 274, 119117. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Tong, T.; Chen, H.-C.; Yu, A.; Yu, J.; Chen, H.M. Single-Atom Engineering of Directional Charge Transfer Channels and Active Sites for Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28, 1802169. [Google Scholar] [CrossRef]
- Chu, C.; Zhu, Q.; Pan, Z.; Gupta, S.; Huang, D.; Du, Y.; Weon, S.; Wu, Y.; Muhich, C.; Stavitski, E.; et al. Spatially Separating Redox Centers on 2D Carbon Nitride with Cobalt Single Atom for Photocatalytic H2O2 Production. Pans 2020, 117, 6376–6382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.; Wan, K.; Sui, X.; Zhao, N.; Li, H.; Lei, W.; Yu, J.; Liu, X.; Shi, X.; Zhai, M.; et al. Anchoring Single Pt Atoms and Black Phosphorene Dual Co-Catalysts on CdS Nanospheres to Boost Visible-Light Photocatalytic H2 Evolution. Nano Today 2021, 37, 101080. [Google Scholar] [CrossRef]
- Su, H.; Liu, M.; Cheng, W.; Zhao, X.; Hu, F.; Liu, Q. Heterogeneous Single-Site Synergetic Catalysis for Spontaneous Photocatalytic Overall Water Splitting. J. Mater. Chem. A 2019, 7, 11170–11176. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z.; Yang, G.; Kong, F.; Yan, T.; Chen, J.; Huang, B.; An, C.; et al. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, Z. Single Mo Atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef]
- Neubert, S.; Mitoraj, D.; Shevlin, S.A.; Pulisova, P.; Heimann, M.; Du, Y.; Goh, G.K.L.; Pacia, M.; Kruczała, K.; Turner, S.; et al. Highly Efficient Rutile TiO2 Photocatalysts with Single Cu(2) and Fe(3) Surface Catalytic Sites. J. Mater. Chem. A 2016, 4, 3127–3138. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Chen, S.; Luo, Q.; Yan, H.; Lin, Y.; Liu, W.; Cao, L.; Lu, J.; Yang, J.; Yao, T.; et al. Atomic-Level Insight into Optimizing the Hydrogen Evolution Pathway over a Co1-N4 Single-Site Photocatalyst. Angew. Chem. Int. Ed. Engl. 2017, 56, 12191–12196. [Google Scholar] [CrossRef]
- Yang, S.; Pattengale, B.; Lee, S.; Huang, J. Real-Time Visualization of Active Species in a Single-Site Metal-Organic Framework Photocatalyst. ACS Energy Lett. 2018, 3, 532–539. [Google Scholar] [CrossRef]
- Huang, P.; Huang, J.; Pantovich, S.A.; Carl, A.D.; Fenton, T.G.; Caputo, C.A.; Grimm, R.L.; Frenkel, A.I.; Li, G. Selective CO2 Reduction Catalyzed by Single Cobalt Sites on Carbon Nitride under Visible-Light Irradiation. J. Am. Chem. Soc. 2018, 140, 16042–16047. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, S.; Kim, M.; Sinha, A.K.; Lee, S.C.; Jung, E.; Chang, W.J.; Lee, K.S.; Kim, J.H.; Cho, S.P.; et al. Reversible and Cooperative Photoactivation of Single-Atom Cu/TiO2 Photocatalysts. Nat. Mater. 2019, 18, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Ji, W.-B.; Hu, B.-C.; Liang, H.-W.; Xu, X.-X.; Yu, Z.-L.; Li, B.-Y.; Yu, S.-H. Partially Oxidized Ni Nanoparticles Supported on Ni-N Co-doped Carbon Nanofibers As Bifunctional Electrocatalysts for Overall Water Splitting. Nano Energy 2018, 51, 286–293. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Zhang, L.; Si, R.; Shen, M.; Wang, M.; Tian, J.; Shi, J. Electron Configuration Modulation of Nickel Single Atoms for Elevated Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2020, 59, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, Q.; Shi, L.; Yao, Q.; Wang, X.; Yu, A.; Chen, Z.; Fu, Y. Merging Single-Atom-Dispersed Iron and Graphitic Carbon Nitride to a Joint Electronic System for High-Efficiency Photocatalytic Hydrogen Evolution. Small 2019, 15, e1905166. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A Universal Ligand Mediated Method for Large Scale Synthesis of Transition Metal Single Atom Catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical Synthesis of Single Atomic Site Catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef]
- Xia, T.; Long, R.; Gao, C.; Xiong, Y. Design of Atomically Dispersed Catalytic Sites for Photocatalytic CO2 Reduction. Nanoscale 2019, 11, 11064–11070. [Google Scholar] [CrossRef]
- He, T.; Chen, S.; Ni, B.; Gong, Y.; Wu, Z.; Song, L.; Gu, L.; Hu, W.; Wang, X. Zirconium-Porphyrin-Based Metal-Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms. Angew. Chem. Int. Ed. Engl. 2018, 57, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, J.; Li, S.; Huang, C.; Yao, W.; Chen, W.; Zeng, T.; Wu, Q.; Xu, Q. Single Nickel Atoms Anchored on Nitrogen-Doped Graphene as a Highly Active Cocatalyst for Photocatalytic H2 Evolution. ACS Catal. 2018, 8, 11863–11874. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, J.; Wang, R.; Wang, Z.D.; Zhu, Y.; Ren, X.; Li, S.; Dong, X.Y.; Zang, S.Q. Inter-Chain Double-Site Synergistic Photocatalytic Hydrogen Evolution in Robust Cuprous Coordination Polymers. Chem. Commun. 2020, 56, 6261–6264. [Google Scholar] [CrossRef] [PubMed]
- Su, D.W.; Ran, J.; Zhuang, Z.W.; Chen, C.; Qiao, S.Z.; Li, Y.D.; Wang, G.X. Atomically Dispersed Ni in Cadmium-Zinc Sulfide Quantum Dots for High-Performance Visible-Light Photocatalytic Hydrogen Production. Sci. Adv. 2020, 6, eaaz8447. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Li, Y.; Cui, X.; Zhang, Q.; Xie, Z.; Liu, H.; Feng, Y.; Lv, W.; Liu, G. The Facile Synthesis of a Single Atom-Dispersed Silver-Modified Ultrathin g-C3N4 Hybrid for the Enhanced Visible-Light Photocatalytic Degradation of Sulfamethazine With Peroxymonosulfate. Dalton Trans. 2018, 47, 6924–6933. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-W.; Chen, S.-M.; Wang, H.-J.; Zhang, Z.-M.; Lin, H.; Song, L.; Lu, T.-B. Single-Atom Molybdenum Immobilized on Photoactive Carbon Nitride as Efficient Photocatalysts for Ambient Nitrogen Fixation in Pure Water. J. Mater. Chem. A 2019, 7, 19831–19837. [Google Scholar] [CrossRef]
- Du, X.L.; Wang, X.L.; Li, Y.H.; Wang, Y.L.; Zhao, J.J.; Fang, L.J.; Zheng, L.R.; Tong, H.; Yang, H.G. Isolation of Single Pt Atoms in a Silver Cluster: Forming Highly Efficient Silver-Based Cocatalysts for Photocatalytic Hydrogen Evolution. Chem. Commun. 2017, 53, 9402–9405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, F.; Li, N.; Zhang, Y.; Mu, Z.; Tang, Y.; Lai, J.; Chao, Y.; Luo, M.; Lin, F.; et al. Strengthening Reactive Metal-Support Interaction to Stabilize High-Density Pt Single Atoms on Electron-Deficient g-C3N4 for Boosting Photocatalytic H2 Production. Nano Energy 2019, 56, 127–137. [Google Scholar] [CrossRef]
- Yu, H.; Cao, C.; Wang, X.; Yu, J. Ag-Modified BiOCl Single-Crystal Nanosheets: Dependence of Photocatalytic Performance on the Region-Selective Deposition of Ag Nanoparticles. J. Phys. Chem. C 2017, 121, 13191–13201. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced Photocatalytic H2 Production Activity of Anatase TiO2 Nanosheet by Selectively Depositing Du-Al-Cocatalysts on {101} and {001} Facets. Appl. Catal. B 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P.C. Atomic Layer Deposition-Sequential Self-Limiting Surface Reactions for Advanced Catalyst “Bottom-Up” Synthesis. Surf. Sci. Rep. 2016, 71, 410–472. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Lin, Y.; Huang, L.; Sun, Z.; Yang, Y.; Zhou, X.; Vovk, E.; Liu, X.; Huang, X.; Sun, M.; et al. Copper Catalysts in Semihydrogenation of Acetylene: From Single Atoms to Nanoparticles. ACS Catal. 2020, 10, 3495–3504. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum Single-Atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Jin, Y.; Wu, T.; Ma, L.; Liang, X. Supported Single Fe Atoms Prepared via Atomic Layer Deposition for Catalytic Reactions. ACS Appl. Nano Mater. 2020, 3, 2867–2874. [Google Scholar] [CrossRef]
- Zeng, Z.; Su, Y.; Quan, X.; Choi, W.; Zhang, G.; Liu, N.; Kim, B.; Chen, S.; Yu, H.; Zhang, S. Single-Atom Platinum Confined by the Interlayer Nanospace of Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution. Nano Energy 2020, 69, 104409. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, D.; Lin, Y.; Liu, W.; Cao, L.; Liu, X.; Zhang, W.; Mou, X.; Fang, S.; Shen, X.; et al. Single Pt Atom with Highly Vacant d-Orbital for Accelerating Photocatalytic H2 Evolution. ACS Appl. Energ. Mater. 2018, 1, 6082–6088. [Google Scholar] [CrossRef]
- Su, H.; Che, W.; Tang, F.; Cheng, W.; Zhao, X.; Zhang, H.; Liu, Q. Valence Band Engineering via PtII Single-Atom Confinement Realizing Photocatalytic Water Splitting. J. Phys. Chem. C 2018, 122, 21108–21114. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, C.; Wang, X. Dispersing Molecular Cobalt in Graphitic Carbon Nitride Frameworks for Photocatalytic Water Oxidation. Small 2015, 11, 1215–1221. [Google Scholar] [CrossRef]
- Xue, Y.; Lei, Y.; Liu, X.; Li, Y.; Deng, W.; Wang, F.; Min, S. Highly Active Dye-Sensitized Photocatalytic H2 Evolution Catalyzed by a Single-Atom Pt Cocatalyst Anchored Onto g-C3N4 Nanosheets under Long-Wavelength Visible Light Irradiation. New, J. Chem. 2018, 42, 14083–14086. [Google Scholar] [CrossRef]
- Yi, L.; Lan, F.; Li, J.; Zhao, C. Efficient Noble-Metal-Free Co-NG/TiO2 Photocatalyst for H2 Evolution: Synergistic Effect between Single-Atom Co and N-Doped Graphene for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 12766–12775. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, H.; Qu, J.; Xia, B.; Zhang, X.; Chen, S.; Song, L.; Jing, L.; Zheng, R.; Qiao, S.Z. Atomically Dispersed Single Co Sites in Zeolitic Imidazole Frameworks Promoting High-Efficiency Visible-Light-Driven Hydrogen Production. Chem.-Eur. J. 2019, 25, 9670–9677. [Google Scholar] [CrossRef]
- Cai, S.; Wang, L.; Heng, S.; Li, H.; Bai, Y.; Dang, D.; Wang, Q.; Zhang, P.; He, C. Interaction of Single-Atom Platinum–Oxygen Vacancy Defects for the Boosted Photosplitting Water H2 Evolution and CO2 Photoreduction: Experimental and Theoretical Study. J. Phys. Chem. C 2020, 124, 24566–24579. [Google Scholar] [CrossRef]
- Jiang, X.H.; Zhang, L.S.; Liu, H.Y.; Wu, D.S.; Wu, F.Y.; Tian, L.; Liu, L.L.; Zou, J.P.; Luo, S.L.; Chen, B.B. Silver Single Atom in Carbon Nitride Catalyst for Highly Efficient Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2020, 59, 23112–23116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zuo, S.; Qiu, M.; Wang, S.; Zhang, Y.; Zhang, J.; Lou, X.W.D. Direct Probing of Atomically Dispersed Ru Species Over Multi-Edged TiO2 for Highly Efficient Photocatalytic Hydrogen Evolution. Sci. Adv. 2020, 6, eabb9823. [Google Scholar] [CrossRef]
- Wu, X.; Zuo, S.; Qiu, M.; Li, Y.; Zhang, Y.; An, P.; Zhang, J.; Zhang, H.; Zhang, J. Atomically Defined Co on Two-Dimensional TiO2 Nanosheet for Photocatalytic Hydrogen Evolution. Chem. Eng. J 2021, 420, 127681. [Google Scholar] [CrossRef]
- Zuo, Q.; Feng, K.; Zhong, J.; Mai, Y.; Zhou, Y. Single-Metal-Atom Polymeric Unimolecular Micelles for Switchable Photocatalytic H2 Evolution. CCS Chemistry 2021, 3, 1963–1971. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Bai, B.; Liu, J.; Zhang, J. Atomically Dispersed Catalytic Sites: A New Frontier for Cocatalyst/Photocatalyst Composites toward Sustainable Fuel and Chemical Production. Catalysts 2021, 11, 1168. https://doi.org/10.3390/catal11101168
Zhang S, Bai B, Liu J, Zhang J. Atomically Dispersed Catalytic Sites: A New Frontier for Cocatalyst/Photocatalyst Composites toward Sustainable Fuel and Chemical Production. Catalysts. 2021; 11(10):1168. https://doi.org/10.3390/catal11101168
Chicago/Turabian StyleZhang, Shuping, Bing Bai, Jia Liu, and Jiatao Zhang. 2021. "Atomically Dispersed Catalytic Sites: A New Frontier for Cocatalyst/Photocatalyst Composites toward Sustainable Fuel and Chemical Production" Catalysts 11, no. 10: 1168. https://doi.org/10.3390/catal11101168
APA StyleZhang, S., Bai, B., Liu, J., & Zhang, J. (2021). Atomically Dispersed Catalytic Sites: A New Frontier for Cocatalyst/Photocatalyst Composites toward Sustainable Fuel and Chemical Production. Catalysts, 11(10), 1168. https://doi.org/10.3390/catal11101168