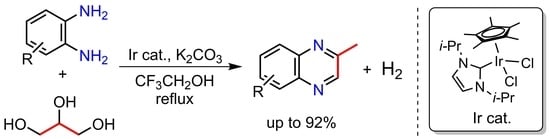
Synthesis of 2-Methylquinoxaline Derivatives from Glycerol and Diamines Catalyzed by Iridium Complexes Bearing an N-Heterocyclic Carbene Ligand
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for Optimization of the Reaction Conditions for the Synthesis of 2-Methyquinoxaline (2a)
3.3. General Procedure for the Synthesis of 2-Methylquinoxaline Derivatives
3.4. Procedure for Quantitative Analysis of the Evolved Hydrogen along with the Formation of 2a
3.5. Procedure for the Synthesis of 2a by the Catalytic Reaction of 1a with Glyceraldehyde
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References and Notes
- Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaxelaire, C.; Winter, P.; Christmann, M. One-Pot Reactions Accelerate the Synthesis of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2011, 50, 3605–3607. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Röder, A.; Krause, N. Synthesis and Properties of Allenic Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2004, 43, 1196–1216. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful Fluorine-Containing Herbicide Agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Dailey, S.; Feast, W.J.; Peace, R.J.; Sage, I.C.; Till, S.; Wood, E.L. Synthesis and Device Characterisation of Side-Chain Polymer Electron Transport Materials for Organic Semiconductor Applications. J. Mater. Chem. 2001, 11, 2238–2243. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for Organic Transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Nadres, E.T.; Daugulis, O. Heterocycle Synthesis via Direct C-H/N-H Coupling. J. Am. Chem. Soc. 2012, 134, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Multicomponent Reactions for the Synthesis of Pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef]
- Hill, M.D. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem. Eur. J. 2010, 16, 12052–12062. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic Cascade Reactions as a New Tool in Total Synthesis. Nature. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Fang, Z.; Aida, T.M.; Smith, R.L. Cycloamination Strategies for Renewable N-Heterocycles. Green Chem. 2020, 22, 582–611. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.A.I.; Fathalla, W. N1-Allyl-3-substituted-6,7-dimethyl-1, 2-dihydro-2-quinoxalinone as Key Intermediate for New Acyclonucleosides and Their Regioisomer O-Analogues. Heteroatom Chem. 2006, 17, 280–288. [Google Scholar] [CrossRef]
- Barker, A.J.; Hamilton, E. New Hypoxia-selective Cytotoxines Derived from Quinoxaline 1,4-Dioxides. J. Heterocycl. Chem. 1995, 32, 1213–1217. [Google Scholar]
- Kaushal, T.; Srivastava, G.; Sharma, A.; Negi, A.S. An Insight into Medicinal Chemistry of Anticancer Quinoxalines. Bioorg. Med. Chem. 2019, 27, 16–35. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective Catalytic Conversion of Glycerol as a Biorenewable Source to Valuable Commodity Chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Xiao, G. Sustainable Value-Added C3 Chemicals from Glycerol Transformations: A Mini Review for Heterogeneous Catalytic Processes. Chin. J. Chem. Eng. 2019, 27, 1536–1542. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Jiang, H.; Chen, M.; Lv, W.; Zheng, A.; Jian, X. Efficient Synthesis of Quinoxalines from 2-Nitroanilines and Vicinal Diols via a Ruthenium-Catalyzed Hydrogen Transfer Strategy. Green Chem. 2015, 17, 279–284. [Google Scholar] [CrossRef]
- Hille, T.; Irrgang, T.; Kempe, R. The Synthesis of Benzimidazoles and Quinoxalines from Aromatic Diamines and Alcohols by Iridium-Catalyzed Acceptorless Dehydrogenative Alkylation. Chem. Eur. J. 2014, 20, 5569–5572. [Google Scholar] [CrossRef]
- Lv, D.; Xie, Z.; Gu, B.; Wu, H.; Wan, H. Highly Efficient Synthesis of Quinoxaline Derivatives Catalized by Iridium Complex. Russ. J. Gen. Chem. 2016, 86, 2887–2890. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Maji, M.; Kundu, S. Cooperative Iridium Complex-Catalyzed Synthesis of Quinoxalines, Benzimidazoles and Quinazolines in Water. Green Chem. 2019, 21, 1999–2004. [Google Scholar] [CrossRef]
- The borrowing-hydrogen process is a catalytic method that diversifies the use of alcohols in organic synthesis. First, catalytic elimination of hydrogen from the alcohol proceeds, producing a carbonyl compound. Subsequently, the condensation reaction of the carbonyl compound produces various unsaturated intermediates. Then, the unsaturated intermediate catalytically receives the “borrowed” hydrogen in the first step, giving the final product. In this way, alcohol can be used as an alkylating agent in borrowing-hydrogen reactions, and the only by-product is water. Examples of the catalytic reactions based on the borrowing-hydrogen process are cited in references [26,27,28, 29]: Fujita, K.; Enoki, Y.; Yamaguchi, R. Cp*Ir-Catalyzed N-Alkylation of Amines with Alcohols. A Versatile and Atom Economical Method for the Synthesis of Amines. Tetrahedron 2008, 64, 1943–1954.
- Fujita, K.; Fujii, T.; Yamaguchi, R. Cp*Ir Complex-Catalyzed N-Heterocyclization of Primary Amines with Diols: A New Catalytic System for Environmentally Benign Synthesis of Cyclic Amines. Org. Lett. 2004, 20, 3525–3528. [Google Scholar] [CrossRef]
- Toyooka, G.; Tuji, A.; Fujita, K. Efficient and Versatile Catalytic Systems for the N-Methylation of Primary Amines with Methanol Catalyzed by N-Heterocyclic Carbene Complexes of Iridium. Synthesis 2018, 50, 4617–4626. [Google Scholar]
- Enomoto, A.; Shimbayashi, T.; Fujita, K. Environmentally Friendly Synthesis of N-Methylated Nitrogen Heterocycles from an Aqueous Solution of Methylamine and Diols. Heterocycles 2019, 98, 1119–1129. [Google Scholar]
- Appayee, C.; Breslow, R. Deuterium Studies Reveal a New Mechanism for the Formose Reaction Involving Hydride Shifts. J. Am. Chem. Soc. 2014, 136, 3720–3723. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Martínz-Silvestre, S. Gold Catalysis Opens Up a New Route for the Synthesis of Benzimidazoylquinoxaline Derivative from Biomass-Derived Products (Glycerol). ChemCatChem 2013, 5, 3866–3874. [Google Scholar] [CrossRef]
- The yield of hydrogen exceeded the yield of 2a because the dehydrogenation of glycerol alone by catalyst E would proceed to some extent, producing hydrogen.
- Ball, R.G.; Graham, W.A.G.; Heinekey, D.M.; Hoyano, J.K.; McMaster, A.D.; Mattson, B.M.; Michel, S.T. Synthesis and Structure of [(η-C5Me5)Ir(CO)]2. Inorg. Chem. 1990, 29, 2023–2025. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Cooperative Catalysis by Iridium Complexes with a Bipyridonate Ligand: Versatile Dehydrogenative Oxidation of Alcohols and Reversible Dehydrogenation-Hydrogenation between 2-Propanol and Acetone. Angew. Chem. Int. Ed. 2012, 51, 12790–12794. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Multialkylation of Aqueous Ammonia with Alcohols Catalyzed by Water-Soluble Cp*Ir-Ammine Complexes. J. Am. Chem. Soc. 2010, 132, 15108–15111. [Google Scholar] [CrossRef]
- Fujita, K.; Furukawa, S.; Morishima, N.; Shimizu, M.; Yamaguchi, R. N-Alkylation of Aqueous Ammonia with Alcohols Leading to Primary Amines Catalyzed by Water-Soluble N-Heterocyclic Carbene Complexes of Iridium. ChemCatChem 2018, 10, 1993–1997. [Google Scholar] [CrossRef]
- Tanabe, Y.; Hanasaka, F.; Fujita, K.; Yamaguchi, R. Scope and Mechanistic Studies of Intramolecular Aliphatic C-H Bond Activation of N-Heterocyclic Carbene Iridium Complexes. Organometallics 2007, 26, 4618–4626. [Google Scholar] [CrossRef]
- Xiao, X.-Q.; Jin, G.-X. Functionalized N-Heterocyclic Carbene Iridium Complexes: Synthesis, Structure and Addition Polymerization of Norbornene. J. Organomet. Chem. 2008, 693, 3363–3368. [Google Scholar] [CrossRef]
- Sakai, H.; Shinto, S.; Araki, Y.; Wada, T.; Sakanoue, T.; Takenobu, T.; Hasobe, T. Formation of One-Dimensional Helical Columns and Excimerlike Excited States by Racemic Quinoxaline-Fused [7]Carbohelicenes in the Crystal. Chem. Eur. J. 2014, 20, 10099–10109. [Google Scholar] [CrossRef]
- Imanishi, M.; Sonoda, M.; Miyazato, H.; Sugimoto, K.; Akagawa, M.; Tanimori, S. Sequential Synthesis, Olfactory Properties, and Biological Activity of Quinoxaline Derivatives. ACS Omega 2017, 2, 1875–1885. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, F.; Shu, W.-M.; Wu, A.-X. Copper-Catalyzed Domino Synthesis of 2-Imino-1H-imidazol-5(2H)-ones and Quinoxalines Involving C-C Bond Cleavage with a 1,3-Dicarbonyl Unit as a Leaving Group. Chem. Eur. J. 2014, 20, 11776–11782. [Google Scholar] [CrossRef]







| Entry | Ir Catalyst | Yield of 2a (%)a |
|---|---|---|
| 1 | none | 0 |
| 2 | [Cp*IrCl2]2 | trace |
| 3 | A | 13 |
| 4 | B | trace |
| 5 | C | 67 |
| 6 | D | 68 |
| 7 | E | 77 |
| 8 | F | 41 |
| 9 | G | 10 |

| Entry | Base (mmol) | Solvent | Yield of 2a (%) a |
|---|---|---|---|
| 1 | none | CF3CH2OH | 2 |
| 2 | Na2CO3 (0.50) | CF3CH2OH | 27 |
| 3 | Li2CO3 (0.50) | Li2CO3 (0.50) | 0 |
| 4 | Cs2CO3 (0.50) | CF3CH2OH | 75 |
| 5 | K2CO3 (0.50) | CF3CH2OH | 82 |
| 6 | K2CO3 (1.0) | CF3CH2OH | 92 (88 b) |
| 7 | KOH (1.0) | CF3CH2OH | 70 |
| 8 | K2CO3 (1.0) | toluene | trace |
| 9 | K2CO3 (1.0) | anisole | 9 |
| 10 | K2CO3 (1.0) | H2O | 21 |
| 11 | K2CO3 (1.0) | t-BuOH | 1 |

| Entry | Substrate (1) | Product (2) | Ratio of 2A : 2B a | Yield of 2 (%) a |
|---|---|---|---|---|
| 1 |  |  | 88 | |
| 2 |  |  | 60 : 40 | 88 |
| 3 b |  |  | 79 : 21 | 82 |
| 4 c |  |  | 15 : 85 | 58 d |
| 5 |  |  | 74 | |
| 6 b |  |  | 65 | |
| 7 b |  |  | 55 | |
| 8 b |  |  | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Enomoto, A.; Furukawa, S.; Fujita, K.-i. Synthesis of 2-Methylquinoxaline Derivatives from Glycerol and Diamines Catalyzed by Iridium Complexes Bearing an N-Heterocyclic Carbene Ligand. Catalysts 2021, 11, 1200. https://doi.org/10.3390/catal11101200
Tanaka T, Enomoto A, Furukawa S, Fujita K-i. Synthesis of 2-Methylquinoxaline Derivatives from Glycerol and Diamines Catalyzed by Iridium Complexes Bearing an N-Heterocyclic Carbene Ligand. Catalysts. 2021; 11(10):1200. https://doi.org/10.3390/catal11101200
Chicago/Turabian StyleTanaka, Toshiki, Akane Enomoto, Shohichi Furukawa, and Ken-ichi Fujita. 2021. "Synthesis of 2-Methylquinoxaline Derivatives from Glycerol and Diamines Catalyzed by Iridium Complexes Bearing an N-Heterocyclic Carbene Ligand" Catalysts 11, no. 10: 1200. https://doi.org/10.3390/catal11101200
APA StyleTanaka, T., Enomoto, A., Furukawa, S., & Fujita, K. -i. (2021). Synthesis of 2-Methylquinoxaline Derivatives from Glycerol and Diamines Catalyzed by Iridium Complexes Bearing an N-Heterocyclic Carbene Ligand. Catalysts, 11(10), 1200. https://doi.org/10.3390/catal11101200