Screening of Acetyl Donors and the Robust Enzymatic Synthesis of Acetyl-CoA by 10-Deacetylbaccatin III-10-β-O-acetyltransferase
Abstract
:1. Introduction
2. Results
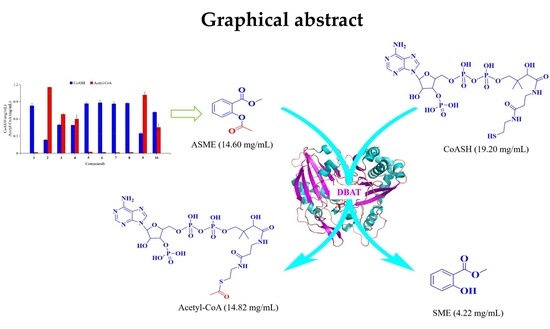
2.1. Screening of Acetyl Donors
2.2. Optimization of Conditions for Synthesis of Acetyl-CoA with ASME as the Acetyl Donor
2.3. Re-Optimization by Increasing the Concentrations of CoASH/ASME and DBAT
2.4. Kinetic Study of DBAT
2.5. Preparation of Acetyl-CoA
2.6. The 1H NMR Data of Acetyl-CoA
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Chemicals and Media
4.3. Screening of Acetyl Donors and Optimization of Reaction Conditions
4.4. Enzyme Kinetic Analysis
4.5. Purification of Acetyl-CoA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Liu, Y.; Yang, Y.; Wang, S.; Wang, Q.; Wang, X.; Yan, Z.; Cheng, J.; Liu, C.; Yang, X.; et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design. Nat. Commun. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Wilson, I.B. Preparation of acetyl coenzyme A1. J. Am. Chem. Soc. 1952, 74, 3205–3206. [Google Scholar] [CrossRef]
- Clough, R.C.; Barnum, S.R.; Jaworski, J. Synthesis of radiolabeled acetyl-coenzyme A from sodium acetate. Anal. Biochem. 1989, 176, 82–84. [Google Scholar] [CrossRef]
- Ouyang, T.; Walt, D.-R. A new chemical method for synthesizing and recycling acyl coenzyme A thioesters. J. Org. Chem. 1991, 56, 3752–3755. [Google Scholar] [CrossRef]
- Rajgarhia, V.; Priestley, N.; Strohl, W. Efficient synthesis of radiolabeled propionyl-coenzyme A and acetyl-coenzyme, A. Anal. Biochem. 1995, 224, 159–162. [Google Scholar] [CrossRef]
- Mishra, P.K.; Drueckhammer, D.G. Coenzyme A analogues and derivatives: Synthesis and applications as mechanistic probes of coenzyme A ester-utilizing enzymes. Chem. Rev. 2000, 100, 3283–3310. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Novelli, G.D.; Lipmann, F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J. Biol. Chem. 1951, 191, 365–376. [Google Scholar] [CrossRef]
- Fritz, I.B.; Schultz, S.K.; Srere, P.A. Properties of partially purified carnitine acetyltransferase. J. Biol. Chem. 1963, 238, 2509–2517. [Google Scholar] [CrossRef]
- Huang, K. A sensitive assay method of acetyl CoA synthetase. Anal. Biochem. 1970, 37, 98–104. [Google Scholar] [CrossRef]
- Pfitzner, A.; Kubicek, C.P.; Röhr, M. Presence and regulation of ATP: Citrate lyase from the citric acid producing fungus Aspergillus Niger. Arch. Microbiol. 1987, 147, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Camp, P.J.; Randall, D.D. Purification and characterization of the pea chloroplast pyruvate dehydrogenase complex. Plant Physiol. 1985, 77, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, B.-Y.; Gong, T.; Chen, T.-J.; Chen, J.-J.; Yang, J.-L.; Zhu, P. Construction of acetyl-CoA and DBAT hybrid metabolic pathway for acetylation of 10-deacetylbaccatin III to baccatin III. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Santaladchaiyakit, Y.; Phiroonsoontorn, N.; Sillapatiwat, C.; Kotchalee, K.; Srijaranai, S. Methyl Salicylate: An alternative extraction solvent for dispersive-liquid-liquid microextraction of benzimidazole fungicides in water samples followed by high-performance liquid chromatographic analysis. J. Braz. Chem. Soc. 2015, 26, 2014–2021. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, H.; Strand, R.; Wang, Y. Modulation of partition and localization of perfume molecules in sodium dodecyl sulfate micelles. Soft Matter 2016, 12, 219–227. [Google Scholar] [CrossRef]
- James, T.; Collins, S.; Amlôt, R.; Marczylo, T. GC–MS/MS quantification of benzyl salicylate on skin and hair: A novel chemical simulant for human decontamination studies. J. Chromatogr. B 2019, 1129, 121818. [Google Scholar] [CrossRef] [PubMed]
- Molleti, J.; Yadav, G.D. Green synthesis of methyl salicylate using novel sulfated iron oxide–zirconia catalyst. Clean Technol. Environ. Policy 2018, 21, 533–545. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.-L.; Jiang, X.-J.; Wang, S.-M.; Zhang, J.-W.; Gui, S.-Y. HII mesophase as a drug delivery system for topical application of methyl salicylate. Eur. J. Pharm. Sci. 2017, 100, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Bearne, S.L. Synthesis of coenzyme A thioesters using methyl acyl phosphates in an aqueous medium. Org. Biomol. Chem. 2014, 12, 9760–9763. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Hong, L.L.; Kong, J.Q.; Wang, X.N.; Hong, L.L.; Kong, J.Q. Diacerein as a promising Acyl donor in Biosynthetic Acetyl-CoA and Glycosyl Esters mediated by a multifunctional maltose O-Acetyltransferase from Escherichia coli. J. Agric. Food Chem. 2021, 69, 6623–6635. [Google Scholar] [CrossRef]
- Walker, K.; Croteau, R. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 583–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.K.; Chang, S.H.; Lung, J.; Tsai, C.J.; Chen, K.P. The strategies to increase taxol production by using Taxus mairei cells transformed with TS and DBAT genes. Int. J. Sci. Eng. 2005, 3, 179–185. [Google Scholar] [CrossRef]
- Lin, S.; Wei, T.; Lin, J.-F.; Guo, L.-Q.; Wu, G.-P.; Wei, J.-B.; Huang, J.-J.; Ouyang, P.-L. Bio-production of Baccatin III, an important precursor of paclitaxel by a cost-effective approach. Mol. Biotechnol. 2018, 60, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Subban, K.; Jayabaskaran, C.; Chelliah, J. Biochemical insights into the recombinant 10-deacetylbaccatin III-10-β-O-acetyltransferase enzyme from the Taxol-producing endophytic fungus Lasiodiplodia theobromae. FEMS Microbiol. Lett. 2019, 366, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-J.; Wang, H.; Gong, T.; Chen, J.-J.; Chen, T.-J.; Yang, J.-L.; Zhu, P. Improving 10-deacetylbaccatin III-10-β-O-acetyltransferase catalytic fitness for Taxol production. Nat. Commun. 2017, 8, 15544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GraphPad Prism, Version 5.01; GraphPad Software Inc.: San Diego, CA, USA, 2007.




| Substrates | Vmax (μM/min) | Km (μM) | kcat (min−1) | kcat/Km (min−1μM−1) |
|---|---|---|---|---|
| ASME | 26.56 (±0.75) | 1.59 (±0.15) | 13.08 (±0.0.37) | 8.33 (±1.84) |
| ATR | 22.43 (±2.07) | 4.80 (±1.08) | 11.05 (±1.02) | 2.38 (±0.45 |
| ASEE | 17.36 (±1.55) | 4.07 (±0.93) | 8.55 (±0.77) | 2.11 (±0.37) |
| ASA | 12.76 (±0.64) | 3.79 (±0.50) | 6.29 (±0.32) | 1.68 (±0.32) |
| AOA | 10.61 (±1.09) | 4.35 (±1.12) | 5.23 (±0.54) | 1.24 (±0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.-Y.; Wang, H.; Gong, T.; Chen, T.-J.; Chen, J.-J.; Yang, J.-L.; Zhu, P. Screening of Acetyl Donors and the Robust Enzymatic Synthesis of Acetyl-CoA by 10-Deacetylbaccatin III-10-β-O-acetyltransferase. Catalysts 2021, 11, 1240. https://doi.org/10.3390/catal11101240
Zhang B-Y, Wang H, Gong T, Chen T-J, Chen J-J, Yang J-L, Zhu P. Screening of Acetyl Donors and the Robust Enzymatic Synthesis of Acetyl-CoA by 10-Deacetylbaccatin III-10-β-O-acetyltransferase. Catalysts. 2021; 11(10):1240. https://doi.org/10.3390/catal11101240
Chicago/Turabian StyleZhang, Bo-Yong, Hao Wang, Ting Gong, Tian-Jiao Chen, Jing-Jing Chen, Jin-Ling Yang, and Ping Zhu. 2021. "Screening of Acetyl Donors and the Robust Enzymatic Synthesis of Acetyl-CoA by 10-Deacetylbaccatin III-10-β-O-acetyltransferase" Catalysts 11, no. 10: 1240. https://doi.org/10.3390/catal11101240
APA StyleZhang, B. -Y., Wang, H., Gong, T., Chen, T. -J., Chen, J. -J., Yang, J. -L., & Zhu, P. (2021). Screening of Acetyl Donors and the Robust Enzymatic Synthesis of Acetyl-CoA by 10-Deacetylbaccatin III-10-β-O-acetyltransferase. Catalysts, 11(10), 1240. https://doi.org/10.3390/catal11101240