Regioselective Enzymatic Synthesis of Kojic Acid Monoesters
Abstract
:1. Introduction
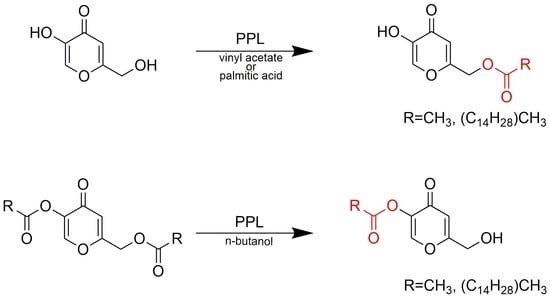
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Kojic Acid Diesters
3.2.1. Kojic Acid Diacetate 4
3.2.2. Kojic Dipalmitate 5
3.3. Enzymatic Reactions
3.3.1. Enzymatic Acetylations of 1
3.3.2. Preparative Enzymatic Acetylation of 1
3.3.3. Enzymatic Deacetylation of Kojic Acid Diacetate 4—General Procedure
3.3.4. Preparative Enzymatic Alcoholysis of 4
3.3.5. Preparative Enzymatic Alcoholysis of Kojic Dipalmitate (5)
3.3.6. Enzymatic Preparation of Kojic Acid 7-Palmitate (7)
3.4. Interpretation of NMR Spectra of Kojic Acid and Its Monoesters and Diesters
- Kojic acid (1, NMR spectra available in Supplementary Materials, Figures S14 and S15)(2-hydroxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 7.95 (s, 1H, H-6), 6.50 (t, J = 0.9 Hz, 1H, H-3), 4.41 (d, J = 0.9 Hz, 1H, CH2-7).13C NMR (101 MHz, CD3OD): δ = 176.8 (C-4), 170.4 (C-2), 147.4 (C-6), 141.0 (C-5), 110.7 (C-3), 61.2 (CH2-7).
- Kojic acid 7-acetate (2)(2-acetoxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 7.98 (s, 1H, H-6), 6.48 (s, 1H, H-3), 4.96 (s, 2H, CH2-7), 2.12 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 176.4 (C-4), 171.6 (COCH3), 164.5 (C-2), 147.7 (C-6), 141.4 (C-5), 113.0 (C-3), 62.5 (CH2-7), 20.4 (COCH3).
- Kojic acid 5-acetate (3)(2-hydroxymethyl-5-acetoxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 8.26 (s, 1H, H-6), 6.57 (t, J = 1.0 Hz, 1H, H-3), 4.46 (d, J = 1.0 Hz, 2H, CH2-7), 2.28 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 175.5 (C-4), 171.7 (COCH3), 169.4 (C-2), 150.9 (C-6), 142.0 (C-5), 113.2 (C-3), 60.9 (CH2-7), 20.1(COCH3).
- Kojic acid 5,7-diacetate (4)(2-acetoxymethyl-5-acetoxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 8.29 (s, 1H, H-6), 6.57 (t, J = 0.7 Hz, 1H, H-3), 5.02 (d, J = 0.7 Hz, 2H, CH2-7), 2.28 (s, 3H, CH3), 2.14 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 175.0 (C-4), 171.5 (COCH3), 169.3 (COCH3), 165.7 (C-2), 151.2 (C-6), 142.2 (C-5), 115.5 (C-3), 62.2 (CH2-7), 20.3 (COCH3), 20.1 (COCH3).
- Kojic Acid 5,7-Dipalmitate (5)(2-palmitoyloxymethyl-5-palmitoyloxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.86 (s, 1H, H-6), 6.47 (s, 1H, H-3), 4.91 (s, 2H, CH2-7), 2.59 (t, J = 7.5 Hz, 2H, CH2-1’), 2.39 (t, J = 7.6 Hz, 2H, CH2-1”), 1.73 (p, J = 7.6 Hz, 2H, CH2-2’), 1.65 (p, J = 7.2 Hz, 2H, CH2-2”), 1.43–1.35 (m, 2H, CH2-3’), 1.33–1.28 (m, 2H, CH2-3”), 1.32 (s, 44H, 22 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3).13C NMR (101 MHz, CDCl3): δ = 172.6 (C-4), 172.3 (COCH2-), 170.7 (COCH2-), 162.5 (C-2), 147.7 (C-6), 141.3 (C-5), 115.1 (C-3), 60.7 (CH2-7), 33.9 (CH2-1’), 33.7 (CH2-1”), 31.9 (CH2-2’, CH2-2”), 29.7 (4 × CH2), 29.7 (2 × CH2), 29.6 (2 × CH2), 29.6 (2 × CH2), 29.6 (CH2), 29.6 (CH2), 29.4 (2 × CH2), 29.4 (2 × CH2), 29.2 (CH2), 29.2 (CH2), 29.1 (CH2), 29.0 (CH2), 24.8 (CH2), 24.7 (CH2), 22.7 (2 × CH2), 14.1 (2 × CH3).
- Kojic Acid 5-Palmitate (6)(2-hydroxymethyl-5-palmitoyloxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.86 (s, 1H, H-6), 6.55 (s, 1H, H-3), 4.49 (s, 2H, CH2-7), 2.59 (t, J = 7.5 Hz, 2H, CH2-1’), 1.73 (p, J = 7.5 Hz, 2H, CH2-2’), 1.35–1.43 (m, 2H, CH2-3’), 1.28–1.33 (m, 2H, CH2-4’), 1.26 (bs, 20H, 10 × CH2), 0.88 (t, J = 6.7 Hz, 3H, CH3).13C NMR (101 MHz, CDCl3): δ = 173.2 (C-4), 170.9 (COCH2-), 168.2 (C-2), 147.8 (C-6), 141.0 (C-5), 113.1 (C-3), 60.7 (CH2-7), 33.6 (CH2-1’), 31.9 (CH2-2’), 29.7 (3 × CH2), 29.6 (3 × CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 29.0 (CH2), 24.7 (CH2), 22.7 (CH2),14.1 (CH3).
- Kojic Acid 7-Palmitate (7)(2-palmitoyloxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.85 (s, 1H, H-6), 6.50 (s, 1H, H-3), 4.93 (s, 2H, CH2-7), 2.40 (t, J = 7.6 Hz, 2H, CH2-1’), 1.65 (p, 2H, J = 7.3 Hz, CH2-2’), 1.34–1.28 (m, 8H, 4 × CH2), 1.26 (bs, 16H, 8 × CH2), 0.88 (t, J = 6.8 Hz, CH3).13C NMR (101 MHz, CDCl3): δ = 174.0 (C-4), 172.7 (COCH2-), 163.0 (C-2), 145.9 (C-6), 138.2 (C-5), 111.2 (C-3), 61.1 (CH2-7), 33.9 (CH2-1’), 31.9 (CH2-2’), 29.7 (3 × CH2), 29.6 (3 × CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 29.1 (CH2), 24.8 (CH2), 22.7 (CH2), 14.1 (CH3).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.H.; Lu, P.J.; Hulme, C.; Shaw, A.Y. Synthesis of kojic acid-derived copper-chelating apoptosis inducing agents. Med. Chem. Res. 2013, 22, 995–1003. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Goh, M.; Lee, J.; Ahn, S.M.; Yeon, J.; Yoo, D.S.; Kim, D.H.; Kim, H.G.; Cho, J.Y. Ester derivatives of kojic acid and polyphenols containing adamantane moiety with tyrosinase inhibitory and anti-inflammatory properties. Bull. Korean Chem. Soc. 2011, 32, 1411–1414. [Google Scholar] [CrossRef] [Green Version]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Ismail, F.; Yamamoto, S.; Yamada, H.; Nakanishi, K. Enzymatic synthesis of galactosylkojic acid with immobilized β -galactosidase from Bacillus circulans. Biosci. Biotechnol. Biochem. 1995, 59, 543–545. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Giridhar, R.; Wu, W.T. Regioselective formation of kojic acid-7-O-alpha-D-glucopyranoside by whole cells of mutated Xanthomonas campestris. Enzyme Microb. Technol. 2007, 40, 324–328. [Google Scholar] [CrossRef]
- Kitao, S.; Sekine, H. Syntheses of two kojic acid glucosides with sucrose phosphorylase from Leuconostoc mesenteroides. Biosci. Biotechnol. Biochem. 1994, 58, 419–420. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Hamada, H. Functional glucosylation of kojic acid and daidzein with the eucalyptus membrane-associated UDP-glucosyltransferase reaction system. J. Biosci. Bioeng. 2001, 92, 469–471. [Google Scholar] [CrossRef]
- Nishimura, T.; Kometani, T.; Takii, H.; Terada, Y.; Okada, S. Acceptor specificity in the glucosylation reaction of Bacillus subtilis X-23 α-amylase towards various phenolic compounds and the structure of kojic acid glucoside. J. Ferment. Bioeng. 1994, 78, 37–41. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nakanishi, K.; Hassan, M.A. Chromatographic separation of galactosylkojic acid. J. Ferment. Bioeng. 1997, 84, 82–85. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, W.; Fu, J.; Li, Z.; Wang, Z.; Lü, P. Double-lipase catalyzed synthesis of kojic dipalmitate in organic solvents. Chem. Res. Chinese Univ. 2017, 33, 903–907. [Google Scholar] [CrossRef]
- Raku, T.; Tokiwa, Y. Regioselective synthesis of kojic acid esters by Bacillus subtilis protease. Biotechnol. Lett. 2003, 25, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Norddin, F.A.A.; Azhar, S.N.A.S.; Ashari, S.E. Evaluation of direct esterification of fatty acid derivative of kojic acid in co-solvent system: A statistical approach. J. Chem. Eng. Process Technol. 2017, 8, 1000331. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Shaw, J. Lipase-catalyzed synthesis of kojic acid esters in organic solvents. J. Am. Oil Chem. Soc. 1998, 75, 1507–1511. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Lipase-catalyzed synthesis of kojic acid derivative in bioreactors and the analysis of its depigmenting and antioxidant activities. Cosmetics 2017, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Comparative study of stirred and fluidized tank reactor for hydroxyl-kojic acid derivatives synthesis and their biological activities. Turk. J. Biochem. 2018, 43, 205–219. [Google Scholar] [CrossRef]
- Kobayashi, T.; Adachi, S.; Nakanishi, K.; Matsuno, R. Semi-continuous production of lauroyl kojic acid through lipase-catalyzed condensation in acetonitrile. Biochem. Eng. J. 2001, 9, 85–89. [Google Scholar] [CrossRef]
- Khamaruddin, N.H.; Basril, M.; Lian, G.E.C.; Salleh, A.B.; Rahman, R.N.Z.R.; Ariff, A.; Mohamad, R.; Awang, R. Enzymatic synthesis and characterization of palm-based kojic acid ester. J. Oil Palm Res. 2008, 20, 461–469. [Google Scholar]
- Jumbri, K.; Al-Haniff Rozy, M.F.; Ashari, S.E.; Mohamad, R.; Basri, M.; Fard Masoumi, H.R. Optimisation and characterisation of lipase catalysed synthesis of a kojic monooleate ester in a solvent-free system by response surface methodology. PLoS ONE 2015, 10, e0144664. [Google Scholar] [CrossRef]
- Ishak, N.; Lajis, A.F.B.; Mohamad, R.; Ariff, A.B.; Mohamed, M.S.; Halim, M.; Wasoh, H. Kinetics and optimization of lipophilic kojic acid derivative synthesis in polar aprotic solvent using lipozyme RMIM and its rheological study. Molecules 2018, 23, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-S.; Liu, K.-J.; Lou, Y.-H.; Shieh, C.-J. Optimisation of kojic acid monolaurate synthesis with lipase PS from Pseudomonas cepacia. J. Sci. Food Agric. 2002, 82, 601–605. [Google Scholar] [CrossRef]
- El-Boulifi, N.; Ashari, S.E.; Serrano, M.; Aracil, J.; Martínez, M. Solvent-free lipase-catalyzed synthesis of a novel hydroxyl-fatty acid derivative of kojic acid. Enzyme Microb. Technol. 2014, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ashari, S.E.; Mohamad, R.; Ariff, A.; Basri, M.; Salleh, A.B. Optimization of enzymatic synthesis of palm-based kojic acid ester using response surface methodology. J. Oleo Sci. 2009, 58, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, G.; Piattelli, M.; Sanfilippo, C. Acetylation of phenols in organic solvent catalyzed by a lipase from Chromobacterium viscosum. Tetrahedron 1992, 48, 2477–2482. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Ma, C.Y.; Lou, Z.X.; Chen, S.W.; Dai, J.; Wang, H.X. Optimization of lipase-catalyzed synthesis of acetylated EGCG by response surface methodology. J. Mol. Catal. B Enzym. 2013, 97, 87–94. [Google Scholar] [CrossRef]
- Peña-Montes, C.; Lange, S.; Castro-Ochoa, D.; Ruiz-Noria, K.; Cruz-García, F.; Schmid, R.; Navarro-Ocaña, A.; Farrés, A. Differences in biocatalytic behavior between two variants of StcI esterase from Aspergillus nidulans and its potential use in biocatalysis. J. Mol. Catal. B Enzym. 2009, 61, 225–234. [Google Scholar] [CrossRef]
- Luddy, F.E.; Barford, R.A.; Herb, S.F.; Magidman, P.; Riemenschneider, R.W. Pancreatic lipase hydrolysis of triglycerides by a semimicro technique. J. Am. Oil Chem. Soc. 1964, 41, 693–696. [Google Scholar] [CrossRef]
- Biely, P.; Côté, G.L.; Kremnický, L.; Weisleder, D.; Greene, R.V. Substrate specificity of acetylxylan esterase from Schizophyllum commune: Mode of action on acetylated carbohydrates. Biochim. Biophys. Acta 1996, 1298, 209–222. [Google Scholar] [CrossRef]









| Alcohol | Tmax (h) 1 | Consumption of 4 (%) | Formation of 3 (%) |
|---|---|---|---|
| dried methanol | 6 | 89.74 | 75.32 |
| 99.0% ethanol | 6 | 87.00 | 73.02 |
| 99.8% n-propanol | 6 | 89.97 | 69.24 |
| 99.8% n-butanol | 6 | 88.50 | 77.06 |
| Substance | H-6 (ppm) | H-7 (ppm) | Solvent |
|---|---|---|---|
| Kojic acid 1 | 7.95 | 4.41 | CD3OD |
| Kojic 7-acetate 2 | 7.98 | 4.96 | CD3OD |
| Kojic 5-acetate 3 | 8.26 | 4.46 | CD3OD |
| Kojic diacetate 4 | 8.29 | 5.02 | CD3OD |
| Kojic dipalmitate 5 | 7.86 | 4.91 | CDCl3 |
| Kojic 5-palmitate 6 | 7.86 | 4.49 | CDCl3 |
| Kojic 7-palmitate 7 | 7.85 | 4.93 | CDCl3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkeszová, K.; Mastihubová, M.; Mastihuba, V. Regioselective Enzymatic Synthesis of Kojic Acid Monoesters. Catalysts 2021, 11, 1430. https://doi.org/10.3390/catal11121430
Karkeszová K, Mastihubová M, Mastihuba V. Regioselective Enzymatic Synthesis of Kojic Acid Monoesters. Catalysts. 2021; 11(12):1430. https://doi.org/10.3390/catal11121430
Chicago/Turabian StyleKarkeszová, Klaudia, Mária Mastihubová, and Vladimír Mastihuba. 2021. "Regioselective Enzymatic Synthesis of Kojic Acid Monoesters" Catalysts 11, no. 12: 1430. https://doi.org/10.3390/catal11121430
APA StyleKarkeszová, K., Mastihubová, M., & Mastihuba, V. (2021). Regioselective Enzymatic Synthesis of Kojic Acid Monoesters. Catalysts, 11(12), 1430. https://doi.org/10.3390/catal11121430