Performance of Liquid Eversa on Fatty Acid Ethyl Esters Production by Simultaneous Esterification/Transesterification of Low-to-High Acidity Feedstocks
Abstract
:1. Introduction
2. Results and Discussion
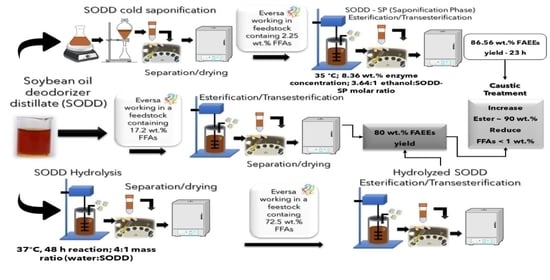
2.1. Soybean Oil Deodorizer Distillate (SODD) Characterization
2.2. SODD Saponification
2.3. SODD Hydrolysis Reaction
2.4. Simultaneous Transesterification and Esterification Reactions
2.5. Performance of Liquid Eversa in Simultaneous Transesterification and Esterification of Fatty Material with Different Free Acidity
3. Materials and Methods
3.1. Materials
3.2. Characterization of the SODD
3.3. SODD Saponification
3.4. SODD Hydrolysis Reaction
3.5. Esterification/Transesterification Reaction Using SODD Saponifiable Phase (SODD-SP) and Ethanol
3.6. Caustic Treatment
3.7. Tocopherol Quantification by Liquid Chromatography
3.8. Quantification of Esters by Gas Chromatography
3.9. Quantification of Glycerol, Triglycerides (TAGs), Diglycerides (DAGs), and Monoglycerides (MAGs) by Gas Chromatography
3.10. Quantification of Free Fatty Acids (FFAs) by Gas Chromatography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| Acyl Donor | Acyl Acceptor | Reaction Conditions | Biocatalyst Form | Yield (%) | Reference |
|---|---|---|---|---|---|
| Soy oil | Ethanol | 6:1 alcohol: oil (molar ratio) 12 U esterification/g oi 40 °C, 1500–1700 rpm, and 24 h | Magnetic CLEAs a | 98.9 | [5] |
| Glyceril trioleate | Fusel oil | 5:1 alcohol:oil (molar ratio) 2 wt.% enzyme 35 °C, 250 rpm, and 24 h | Liquid | >97% | [6] |
| Deacidified cattle tallow | Methanol | 4.5:1 alcohol: oil (molar ratio) 1.0 wt.% enzyme 35 °C, 300 rpm, 6.0 wt.% water, and 8 h | Liquid | 85.08 | [7] |
| Soy oil | Methanol | 550 kg oil and 2.2 kg methanol 0.2 wt.% enzyme, 45 °C, 20.0 wt% water, 100 ppm NaOH, and 24 h | Liquid | 96 | [8] |
| Castor oil | Methanol | 6:1 alcohol: oil (molar ratio) 5.0 wt.% enzyme, 35 °C, 5.0 wt.% water, and 8 h | Liquid | 83 | [9] |
| Residual oil from a poultry industry | Methanol | 100 g oil and 1.5 eqv. alcohol 0.3 wt.% enzyme, 45 °C, 1.5 wt.% water, 250 rpm, and 24 h | Liquid (NS 40116 trademark) | 90.61 | [10] |
| Cotton seed oil | Methanol | 6:1 alcohol: oil (molar ratio) 5 wt.% enzyme, 35 °C, 6 wt.% water, 250 rpm, and24 h | Liquid | 98.5 | [11] |
| Sunflower oil | Ethanol | 1:4 alcohol: oil (molar ratio) 4.1 mL hexane, 10 wt.% enzyme, 40 °C, 150 rpm, and 3 h | Immobilized on Sepabeads | 99 | [12] |
| Oleic acid | Methanol | 3.44:1 alcohol:acid (molar ratio) 11.98% enzyme, 35.25 °C, and 2.5 h | Liquid | 96.73 | [13] |
| CTO b of the kraft pulping process | Methanol | 1.5:1 alcohol: oil (molar ratio) 1 wt.% enzyme, 500 rpm, 40 °C, and 16 h | Liquid | 96.57 | [14] |
| Castor oil | Methanol | 6:1 alcohol: oil (molar ratio) 5 wt.% enzyme, 5 wt.% water, 750 rpm, 35 °C, and 8 h | Liquid | 94.21 | [15] |
| Castor oil | Methanol | 6:1 alcohol: oil (molar ratio) 5 w.t% enzyme, 5 wt.% water, 750 rpm, 35 °C, and 8 h | Liquid | Not informed | [16] |
| Bleached sardine oil | Ethanol | 8:1 alcohol: oil (molar ratio) 60 U enzyme, 10 wt.% water, 25 °C, and 4 h | Liquid | 93.98 | [17] |
| Castor oil | Methanol | 6:1 alcohol: oil (molar ratio) 10 wt.% enzyme, 750 rpm, 35 °C, and 8 h | Liquid | 94.21 | [18] |
| Soy oil | Methanol | 1.5 eqv. methanol 1 wt.% enzyme, 2.5 wt.% water, 250 rpm, 35 °C, and 16 h | Liquid | 96.7 | [19] |
| Soy oil | Methanol | 1.5 eqv. methanol 0.2 wt.% enzyme, 3 wt.% water, 500 rpm, 35 °C, and 24 h | Liquid | 97.5 | [1] |
| SODD-SP c | Ethanol | 3.64:1 ethanol:SODD-SP (molar ratio) 8.36 wt.% enzyme 35 °C and 48 h | Liquid | 90.83 | This study |
| DD Source Oil | Alcohol | Reaction Conditions | Biocatalyst | Yield (%) | Reference |
|---|---|---|---|---|---|
| Soy | Methanol | Methanol:DD 2.3:1 (molar ratio), 53.6 °C, and 2 h | Lipozyme IM a | 88 | [30] |
| Soy | Methanol | Methanol:DD 3.6:1 (molar ratio), 40 °C, and 24 h | Novozym 435 b | 97 | [29] |
| Soy | Methanol | Methanol:DD 3.9:1 (molar ratio), 40 °C, and 24 h | Novozym 435 | 95 | [28] |
| Palm | Ethanol Methanol | 2 g of alcohol added in two steps to 8 g of DD, 60 °C, and 2.5 h | Novozym 435 Lipozyme RM-IM c Lipozyme TL-IM d | 93 | [27] |
| Rapeseed | Ethanol | Ethanol:DD 4:1 (molar ratio), 40 °C, and 30 h | Lipase from Rhizopus oryzae immobilized on hydrophobic macroporous resin NKA e | 98.23 | [26] |
| Rapeseed | Methanol | Methanol:DD 167 µL:2 g, 34 °C, and 6 h | Lipase from Rhizopus oryzae | 98.16 | [25] |
| Factor | SS * | DF * | MS * | F Calculatted | p-Value |
|---|---|---|---|---|---|
| Model | 527.9898 | 9 | 58.6655 | 24.4483 | 0.000176 |
| (1) Molar ratio (ethanol:SODD-SP)(L) | 38.0718 | 1 | 38.0718 | 179.989 | 0.005510 |
| Molar ratio (ethanol:SODD-SP)(Q) | 2.8603 | 1 | 2.8603 | 13.522 | 0.066644 |
| (2) Enzyme concentration (wt.%)(L) | 223.2497 | 1 | 223.2497 | 1055.441 | 0.000946 |
| Enzyme concentration (wt.%)(Q) | 9.9646 | 1 | 9.9646 | 47.109 | 0.020575 |
| (3) Temperature (°C)(L) | 184.3259 | 1 | 184.3259 | 871.424 | 0.001146 |
| Temperature (°C)(Q) | 10.7223 | 1 | 10.7223 | 50.691 | 0.019162 |
| 1L by 2L | 0.9895 | 1 | 0.9895 | 4.678 | 0.163034 |
| 1L by 3L | 54.7595 | 1 | 54.7595 | 258.882 | 0.003841 |
| 2L by 3L | 3.0462 | 1 | 3.0462 | 14.402 | 0.062951 |
| Lack of Fit | 12.0879 | 5 | 2.4176 | 11.429 | 0.082403 |
| Pure Error | 0.4230 | 2 | 0.2115 | ||
| Total SS | 549.5108 | 16 |
References
- Nielsen, P.M.; Rancke-Madsen, A.; Holm, H.C.; Burton, R. Production of biodiesel using liquid lipase formulations. J. Am. Oil Chem. Soc. 2016, 93, 905–910. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244. [Google Scholar] [CrossRef]
- Pedersen, A.T.; Nordblad, M.; Nielsen, P.M.; Woodley, J.M. Batch production of FAEE-biodiesel using a liquid lipase formulation. J. Mol. Catal. B Enzym. 2014, 105, 89–94. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Miranda, L.P.; Guimarães, J.R.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Composites of crosslinked aggregates of Eversa® transform and magnetic nanoparticles. Performance in the ethanolysis of soybean oil. Catalysts 2020, 10, 817. [Google Scholar] [CrossRef]
- Monroe, E.; Shinde, S.; Carlson, J.S.; Eckles, T.P.; Liu, F.; Varman, A.M.; George, A.; Davis, R.W. Superior performance biodiesel from biomass-derived fusel alcohols and low grade oils: Fatty acid fusel esters (FAFE). Fuel 2020, 268, 117408. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Rosset, D.V.; Tres, M.V.; Oliveira, J.V.; Mazutti, M.A.; Jahn, S.L. Production of biodiesel catalyzed by lipase from Thermomyces lanuginosus in its soluble form. Can. J. Chem. Eng. 2018, 96, 2361–2368. [Google Scholar] [CrossRef]
- Mibielli, G.M.; Fagundes, A.P.; Bender, J.P.; Oliveira, J.V. Lab and pilot plant FAME production through enzyme-catalyzed reaction of low-cost feedstocks. Bioresour. Technol. Rep. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Investigation of the use of ceramic membranes in recovering liquid enzymes for castor oil transesterification. Chem. Eng. Trans. 2019, 74, 769–774. [Google Scholar] [CrossRef]
- Coppini, M.; Magro, J.D.; Martello, R.; Valério, A.; Zenevicz, M.C.; De Oliveira, D.; Oliveira, J.V. Production of methyl esters by enzymatic hydroesterification of chicken fat industrial residue. Braz. J. Chem. Eng. 2019, 36, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.; Walker, T.; Moser, B.; Drapcho, C.; Zheng, Y.; Bridges, W. Evaluation of dominant parameters in lipase transesterification of cottonseed oil. Trans. ASABE 2019, 62, 467–474. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via alcoholysis of sunflower oil by Eversa lipases immobilized on hydrophobic supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.-Y.; Su, C.-H.; Chien, C.-C. Liquid lipase-catalyzed esterification of oleic acid with methanol for biodiesel production in the presence of superabsorbent polymer: Optimization by using response surface methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef] [Green Version]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Transesterification of castor oil catalyzed by liquid enzymes: Optimization of reaction conditions. Comput. Aided Chem. Eng. 2017, 40, 2863–2868. [Google Scholar] [CrossRef] [Green Version]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of reaction mechanisms and kinetic parameters for the transesterification of castor oil by liquid enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Castor oil transesterification catalysed by liquid enzymes: Feasibility of reuse under various reaction conditions. Chem. Eng. Trans. 2017, 57, 913–918. [Google Scholar] [CrossRef]
- Remonatto, D.; Santin, C.M.T.; de Oliveira, D.; Di Luccio, M.; de Oliveira, J.V. FAME production from waste oils through commercial soluble lipase Eversa® catalysis. Ind. Biotechnol. 2016, 12, 254–262. [Google Scholar] [CrossRef]
- Sherazi, T.H.S.; Mahesar, A.S. Vegetable oil deodorizer distillate: A rich source of the natural bioactive components. J. Oleo Sci. 2016, 65, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Tufail, S.; Sherazi, H.A.; Talpur, F.N.; Mahesar, S.A.; Kara, H. Rapid determination of free fatty acid content in waste deodorizer distillates using single bounce-attenuated total reflectance-FTIR spectroscopy. J. AOAC Int. 2012, 95, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, S.; Kasim, N.S.; Ju, Y.H. Separation and purification of squalene from soybean oil deodorizer distillate. Sep. Purif. Technol. 2008, 60, 128–135. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Tocopherols and tocotrienols in plants and their products: A review on methods of extraction, chromatographic separation, and detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Franchin, M.; Shahidi, F. Tocopherols and tocotrienols: Sources, analytical methods, and effects in food and biological systems. Encycl. Food Chem. 2019, 561–570. [Google Scholar] [CrossRef]
- Zeng, L.; He, Y.; Jiao, L.; Li, K.; Yan, Y. Preparation of biodiesel with liquid synergetic lipases from rapeseed oil deodorizer distillate. Appl. Biochem. Biotechnol. 2017, 183, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Li, G.; Zhang, H.; Yan, Y. Enhanced performance of Rhizopus oryzae lipase immobilized on hydrophobic carriers and its application in biorefinery of rapeseed oil deodorizer distillate. Bioenergy Res. 2014, 7, 935–945. [Google Scholar] [CrossRef]
- Corrêa, I.N.S.; Lorena de Souza, S.; Catran, M.; Bernardes, O.L.; Portilho, M.F.; Langone, M.A.P. Enzymatic biodiesel synthesis using a byproduct obtained from palm oil refining. Enzyme Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wang, L.; Liu, D. Improved methanol tolerance during Novozym 435-mediated methanolysis of SODD for biodiesel production. Green Chem. 2007, 9, 173–176. [Google Scholar] [CrossRef]
- Wang, L.; Du, W.; Liu, D.; Li, L.; Dai, N. Lipase-catalyzed biodiesel production from soybean oil deodorizer distillate with absorbent present in tert-butanol system. J. Mol. Catal. B Enzym. 2006, 43, 29–32. [Google Scholar] [CrossRef]
- Facioli, N.L.; Barrera-Arellano, D. Optimisation of enzymatic esterification of soybean oil deodoriser distillate. J. Sci. Food Agric. 2001, 81, 1193–1198. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Ma, H.; Dai, C.; Zhang, H.; Li, K.; Li, Y. Biodiesel production from soybean oil deodorizer distillate enhanced by counter-current pulsed ultrasound. Ultrason. Sonochem. 2015, 23, 53–58. [Google Scholar] [CrossRef]
- Yin, X.; Duan, X.; You, Q.; Dai, C.; Tan, Z.; Zhu, X. Biodiesel production from soybean oil deodorizer distillate usingcalcined duck eggshell as catalyst. Energy Convers. Manag. 2016, 112, 199–207. [Google Scholar] [CrossRef]
- Campestre. Óleo de Soja Refinado. Ficha Ténica. Available online: https://www.campestre.com.br/oleos-vegetais/oleo-de-soja/oleo-de-soja-ficha-tecnica/ (accessed on 13 November 2021).
- AOCS. Ca 5a-40. Free Fatty Acids in Crude and Refined Fats and Oils. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS. Cd 1-25. Wijs Method for Iodine Value. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS. Cd 3-25. Saponification Value. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS. Ca 2b-38. Moisture and Volatile Matter in Butter, Fats, Margarines, and Oils, Hot Plate. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 2004. [Google Scholar]
- AOCS. Ce 2-66. Preparation of Methyl Esters of Fatty Acids. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 2004. [Google Scholar]
- Duvekot, C. Determination of Total FAME and Linolenic Acid Methyl Esters in Biodiesel According to EN-14103. Available online: https://www.agilent.com/cs/library/applications/5990-8983EN.pdf (accessed on 18 June 2020).
- AOCS. Ce 8-89. Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 2004. [Google Scholar]
- Agilent Technologies. The Essential Chromatography and Spectroscopy Catalog, 2011/2012. Available online: https://www.agilent.com/en/promotions/catalog (accessed on 1 May 2021).
- Sahu, S.; Ghosh, M.; Bhattacharyya, D.K. Isolation of the unsaponifiable matter (squalene, phytosterols, tocopherols, γ-oryzanol and fatty alcohols) from a fatty acid distillate of rice bran oil. Grasas Aceites 2018, 69, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, L. Biodiesel production from rapeseed deodorizer distillate in a packed column reactor. Chem. Eng. Process. Process Intensif. 2009, 48, 1152–1156. [Google Scholar] [CrossRef]
- Knothe, G. Structure indices in FA chemistry. How relevant is the iodine value? J. Am. Oil Chem. Soc. 2002, 79, 847–854. [Google Scholar] [CrossRef]
- Barros, A.C.; WUST, E.; Meier, H.F. Estudo da viabilidade técnico-científica da produção de biodiesel a partir de resíduos gordurosos. Eng. Sanit. Ambient. 2008, 13, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Brock, J.; Nogueira, M.R.; Zakrzevski, C.; De Castilhos Corazza, F.; Corazza, M.L.; Vladimir De Oliveira, J. Experimental measurements of viscosity and thermal conductivity of vegetable oils. Ciênc. Tecnol. Aliment. Camp. 2008, 28, 564. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, S.; Ju, Y.H. Vegetable oil deodorizer distillate: Characterization, utilization and analysis. Sep. Purif. Rev. 2009, 38, 207–241. [Google Scholar] [CrossRef]
- Kong, W.; Baeyens, J.; De Winter, K.; Urrutia, A.R.; Degrève, J.; Zhang, H. An energy-friendly alternative in the large-scale production of soybean oil. J. Environ. Manag. 2019, 230, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Kasim, N.S.; Gunawan, S.; Yuliana, M.; Ju, Y.H. A simple two-step method for simultaneous isolation of tocopherols and free phytosterols from soybean oil deodorizer distillate with high purity and recovery. Sep. Sci. Technol. 2010, 45, 2437–2446. [Google Scholar] [CrossRef]
- Maniet, G.; Jacquet, N.; Richel, A. Recovery of sterols from vegetable oil distillate by enzymatic and non-enzymatic processes. Comptes Rendus Chim. 2019, 22, 347–353. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Paques, F.W.W.; Macedo, G.A. Lipases de látex vegetais: Propriedades e aplicações industriais. Quim. Nova 2006, 29, 93–99. [Google Scholar] [CrossRef]
- Cavalcanti-Oliveira, E.D.A.; Da Silva, P.R.; Ramos, A.P.; Aranda, D.A.G.; Freire, D.M.G. Study of soybean oil hydrolysis catalyzed by Thermomyces lanuginosus lipase and its application to biodiesel production via hydroesterification. Enzyme Res. 2011, 2011, 618692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; Giordano, R.L.C.; Tardioli, P.W. Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Mibielli, G.M.; Fagundes, A.P.; Bohn, L.R.; Cavali, M.; Bueno, A.; Bender, J.P.; Oliveira, J.V. Enzymatic production of methyl esters from low-cost feedstocks. Biocatal. Agric. Biotechnol. 2020, 24, 101558. [Google Scholar] [CrossRef]
- McCurry, J.D.; Wang, C.-X. Analysis of Glycerin and Glycerides in Biodiesel (B100) Using ASTM D6584 and EN14105. Available online: https://www.agilent.com/cs/library/applications/5989-7269CHCN.pdf (accessed on 18 June 2020).
- Cesarini, S.; Diaz, P.; Nielsen, P.M. Exploring a new, soluble lipase for FAMEs production in water-containing systems using crude soybean oil as a feedstock. Process Biochem. 2013, 48, 484–487. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Tres, M.V.; Jahn, S.L.; Oliveira, J.V. Lipases in liquid formulation for biodiesel production: Current status and challenges. Biotechnol. Appl. Biochem. 2020, 67, 648–667. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Niehus, X.; Casas-Godoy, L.; Sandoval, G. Lipases as biocatalyst for biodiesel production. In Lipases and Phospholipases. Methods in Molecular Biology; Sandoval, G., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1835, pp. 377–390. ISBN 978-1-4939-8672-9. [Google Scholar]
- ANP (Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. Resolução). ANP No 45/2014. Available online: https://ubrabio.com.br/2014/08/26/resolucao-anp-no-45-2014/ (accessed on 2 December 2021).
- AOCS. Cc 10a-25. Specific Gravity of Oils and Liquid Fats. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS. Ca 6a-40. Unsaponifiable Matter in Fats and Oils, Except Marine Oils. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 1990. [Google Scholar]



| Parameter | Results | Method |
|---|---|---|
| Acidity index (mgKOH/g) (25 °C) | 34.19 ± 0.52 (17.18 wt.%) | [34] |
| Iodine index by the Wijs method (gI2/100 g) (25 °C) | 112.98 ± 0.32 | [35] |
| Saponification index (mgKOH/g) (25 °C) | 181.62 ± 0.96 (91.27 wt.%) | [36] |
| Kinematic viscosity (mPa.s) (40 °C) | 32.74 ± 0.01 | Note 1 |
| Kinematic viscosity (mPa.s) (25 °C) | 57.10 ± 0.01 | Note 1 |
| Moisture (%) (130 °C) | 1.33 ± 0.04 | [37] |
| Saponifiable matter as fatty acid methyl esters (FAME) (wt.%) | 85.15 ± 0.53 | [38,39] |
| α-Tocopherol (g/100 g) (%) | 0.37 ± 0.001 | [40] |
| β-Tocopherol (g/100 g) (%) | 0.09 ± 0.005 | [40] |
| γ-Tocopherol (g/100 g) (%) | 1.01 ± 0.003 | [40] |
| δ-Tocopherol (g/100 g) (%) | 0.36 ± 0.003 | [40] |
| Total of tocopherols (g/100 g) (%) | 1.83 | [40] |
| Palmitic acid (C16:0) (g/100 g) (%) | 3.19 ± 0.01 | [41] |
| Stearic acid (C18:0) (g/100 g) (%) | 0.99 ± 0.03 | [41] |
| Oleic acid (C18:1) (g/100 g) (%) | 5.43 ± 0.04 | [41] |
| Linoleic acid (C18:2) (g/100 g) (%) | 7.91 ± 0.15 | [41] |
| Linolenic acid (C18:3) (g/100 g) (%) | 0.95 ± 0.06 | [41] |
| Total of free fatty acids (g/100 g) (%) | 18.47 | [41] |
| Assays | Molar Ratio (Ethanol:SODD-SP) | Enzyme Concentration (wt. %) | Temperature (°C) | Ester Yield Experimental (wt.%) | Ester Yield Predicted (wt.%) |
|---|---|---|---|---|---|
| 1 | 2.3:1 (−1) | 3 (−1) | 30 (−1) | 73.00 ± 2.55 | 72.38 |
| 2 | 3.3:1 (+1) | 3 (−1) | 30 (−1) | 70.77 ± 0.79 | 70.46 |
| 3 | 2.3:1 (−1) | 7 (+1) | 30 (−1) | 81.91 ± 0.80 | 82.40 |
| 4 | 3.3:1 (+1) | 7 (+1) | 30 (−1) | 80.10 ± 1.37 | 80.48 |
| 5 | 2.3:1 (−1) | 3 (−1) | 40 (+1) | 59.30 ± 1.60 | 61.14 |
| 6 | 3.3:1 (+1) | 3 (−1) | 40 (+1) | 69.36 ± 1.03 | 69.70 |
| 7 | 2.3:1 (−1) | 7 (+1) | 40 (+1) | 67.57 ± 1.68 | 67.28 |
| 8 | 3.3:1 (+1) | 7 (+1) | 40 (+1) | 74.40 ± 1.34 | 75.84 |
| 9 | 1.96:1 (−1.68) | 5 (0) | 35 (0) | 67.98 ± 0.72 | 69.70 |
| 10 | 3.64:1 (+1.68) | 5 (0) | 35 (0) | 73.90 ± 2.06 | 75.28 |
| 11 | 2.8:1 (0) | 1.64 (−1.68) | 35(0) | 67.99 ± 4.44 | 68.36 |
| 12 | 2.8:1 (0) | 8.36 (+1.68) | 35 (0) | 82.06 ± 4.87 | 81.93 |
| 13 | 2.8:1 (0) | 5 (0) | 26.6 (−1.68) | 72.94 ± 1.61 | 76.42 |
| 14 | 2.8:1 (0) | 5 (0) | 43.4 (+1.68) | 65.14 ± 4.32 | 63.08 |
| 15 | 2.8:1 (0) | 5 (0) | 35 (0) | 72.03 ± 0.45 | 72.49 |
| 16 | 2.8:1 (0) | 5 (0) | 35 (0) | 72.74 ± 1.01 | 72.49 |
| 17 | 2.8:1 (0) | 5 (0) | 35 (0) | 72.76 ± 0.25 | 72.49 |
| FAEEs Production (Reaction Step) | Caustic Treatment | ||||
|---|---|---|---|---|---|
| Inputs | Outputs | Outputs | |||
| Component | Value (wt.%) | Component | Value (wt.%) | Component | Value (wt.%) |
| Crude SODD | Crude FAEEs | Crude FAEEs | |||
| SI | 91.27 ± 0.88 | Saponifiable | Saponifiable | ||
| FFAs | 17.18 ± 0.26 | FAEEs | 76.85 ± 0.65 | FAEEs | 82.42 ± 0.78 |
| Acyl-gly a | 74.09 | FFAs | 5.08 ± 0.01 | FFAs | 1.08 ± 0.08 |
| Toc-total | 1.83 | Acyl-gly | 1.59 | Acyl-gly | 0.94 |
| α-tocopherol | 0.37 ± 0.001 | Glycerol | 0.04 ± 0.01 | Glycerol | 0.04 ± 0.004 |
| β-tocopherol | 0.09 ± 0.005 | MAGs | 0.73 ± 0.01 | MAGs | 0.42 ± 0.0 |
| γ-tocopherol | 1.01 ± 0.003 | DAGs | 0.79 ± 0.09 | DAGs | 0.45 ± 0.05 |
| δ-tocopherol | 0.36 ± 0.003 | TAGs | 0.03 ± 0.01 | TAGs | 0.03 ± 0.003 |
| Toc-total | 1.39 | Toc-total | 0.41 | ||
| α-tocopherol | 0.06 ± 0.01 | α-tocopherol | 0.002 ± 0.001 | ||
| β-tocopherol | 0.07 ± 0.001 | β-tocopherol | 0.005 ± 0.001 | ||
| γ-tocopherol | 0.99 ± 0.01 | γ-tocopherol | 0.19 ± 0.003 | ||
| δ-tocopherol | 0.27 ± 0.001 | δ-tocopherol | 0.22 ± 0.01 | ||
| SODD–SP | Crude FAEEs | Crude FAEEs | |||
| SI | 93.03 ± 1.08 | Saponifiable | Saponifiable | ||
| FFAs | 2.25 ± 0.13 | FAEEs | 86.56 ± 0.31 | FAEEs | 90.83 ± 0.82 |
| Acyl-gly a | 90.78 | FFAs | 2.77 ± 0.08 | FFAs | 0.82 ± 0.08 |
| Toc-total | 1.33 | Acyl-gly | 1.89 | Acyl-gly | 1.03 |
| α-tocopherol | 0.34 ± 0.01 | Glycerol | 0.05 ± 0.01 | Glycerol | N.d. |
| β-tocopherol | 0.01 ± 0.003 | MAGs | 0.90 ± 0.05 | MAGs | 0.50 ± 0.02 |
| γ-tocopherol | 0.84 ± 0.01 | DAGs | 0.87 ± 0.23 | DAGs | 0.53 ± 0.09 |
| δ-tocopherol | 0.14 ± 0.001 | TAGs | 0.07 ± 0.03 | TAGs | N.d. |
| Toc-total | 1.01 | Toc-total | 0.67 | ||
| α-tocopherol | 0.20 ± 0.05 | α-tocopherol | 0.06 ± 0.02 | ||
| β-tocopherol | 0.01 ± 0.001 | β-tocopherol | 0.01 ± 0.002 | ||
| γ-tocopherol | 0.67 ± 0.02 | γ-tocopherol | 0.49 ± 0.03 | ||
| δ-tocopherol | 0.13 ± 0.001 | δ-tocopherol | 0.11 ± 0.002 | ||
| Hydrolyzed SODD | Crude FAEEs | Crude FAEEs | |||
| SI b | 96.24 | Saponifiable | Saponifiable | ||
| FFAs | 72.48 ± 2.91 | FAEEs | 80.02 ± 0.11 | FAEEs | 88.83 ± 0.85 |
| Acyl-gly | 23.76 | FFAs | 8.07 ± 0.19 | FFAs c | 9.16 ± 0.001 |
| Glycerol | N.d. | Acyl-gly | 11.91 | Acyl-gly | 1.82 |
| MAGs | 8.02 ± 0.05 | Glycerol | N.d. | Glycerol | 0.73 ± 0.08 |
| DAGs | 7.12 ± 0.01 | MAGs | 2.41 ± 0.09 | MAGs | 1.09 ± 0.07 |
| TAGs | 8.62 ± 0.03 | DAGs | 7.64 ± 0.01 | DAGs | N.d. |
| Toc-total | 0.41 | TAGs | 1.86 ± 0.01 | TAGs | N.d. |
| α-tocopherol | 0.10 ± 0.003 | Toc-total | 0.21 | Toc-total | 0.29 |
| β-tocopherol | 0.10 ± 0.08 | α-tocopherol | 0.01 ± 0.005 | α-tocopherol | 0.06 ± 0.05 |
| γ-tocopherol | 0.06 ± 0.01 | β-tocopherol | 0.07 ± 0.003 | β-tocopherol | 0.07 ± 0.004 |
| δ-tocopherol | 0.15 ± 0.02 | γ-tocopherol | 0.03 ± 0.04 | γ-tocopherol | 0.04 ± 0.02 |
| δ-tocopherol | 0.10 ± 0.005 | δ-tocopherol | 0.12 ± 0.01 | ||
| Variables | −1.68 | −1 | 0 | +1 | +1.68 | |
|---|---|---|---|---|---|---|
| Molar ratio (ethanol:SODD-SP) | X1 | 1.96:1 | 2.3:1 | 2.8:1 | 3.3:1 | 3.64:1 |
| Enzyme concentration (wt.%) | X2 | 1.64 | 3 | 5 | 7 | 8.36 |
| Temperature (°C) | X3 | 26.6 | 30 | 35 | 40 | 43.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.C.; Cansian, A.B.M.; Guimarães, J.R.; Vieira, A.M.S.; Fernandez-Lafuente, R.; Tardioli, P.W. Performance of Liquid Eversa on Fatty Acid Ethyl Esters Production by Simultaneous Esterification/Transesterification of Low-to-High Acidity Feedstocks. Catalysts 2021, 11, 1486. https://doi.org/10.3390/catal11121486
Vieira AC, Cansian ABM, Guimarães JR, Vieira AMS, Fernandez-Lafuente R, Tardioli PW. Performance of Liquid Eversa on Fatty Acid Ethyl Esters Production by Simultaneous Esterification/Transesterification of Low-to-High Acidity Feedstocks. Catalysts. 2021; 11(12):1486. https://doi.org/10.3390/catal11121486
Chicago/Turabian StyleVieira, Ana Carolina, Ana Bárbara Moulin Cansian, José Renato Guimarães, Angelica Marquettotti Salcedo Vieira, Roberto Fernandez-Lafuente, and Paulo Waldir Tardioli. 2021. "Performance of Liquid Eversa on Fatty Acid Ethyl Esters Production by Simultaneous Esterification/Transesterification of Low-to-High Acidity Feedstocks" Catalysts 11, no. 12: 1486. https://doi.org/10.3390/catal11121486
APA StyleVieira, A. C., Cansian, A. B. M., Guimarães, J. R., Vieira, A. M. S., Fernandez-Lafuente, R., & Tardioli, P. W. (2021). Performance of Liquid Eversa on Fatty Acid Ethyl Esters Production by Simultaneous Esterification/Transesterification of Low-to-High Acidity Feedstocks. Catalysts, 11(12), 1486. https://doi.org/10.3390/catal11121486