Insights into the Influence of Key Preparation Parameters on the Performance of MoS2/Graphene Oxide Composites as Active Materials in Supercapacitors
Abstract
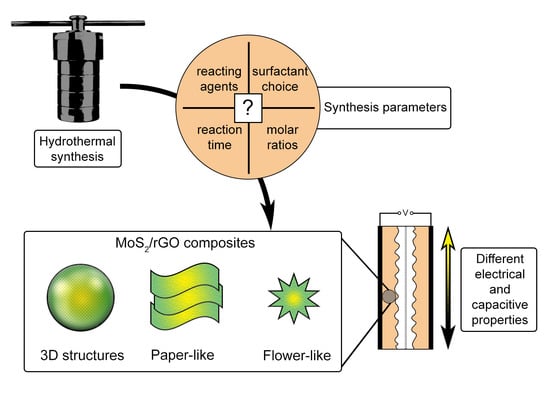
:1. Introduction
2. Synthesis Methods for MoS2/MoS2 Composites
2.1. Top-Down Methods for MoS2 Synthesis
2.2. Bottom-Up Methods
3. The Influence of Preparation Parameters on Morphology, Structure and Surface Particularities of MoS2/MoS2-rGO Composites for Designing Efficient Supercapacitors
3.1. Influence of the Reaction Time in MoS2 and Its Composites Synthesis
3.2. The Influence of Surfactants for Synthesising MoS2 and Its Composites
3.3. The Influence of Reducing Agents for the Synthesis of MoS2 and Its Composites
3.4. The Influence of the Molar Ratios of Molybdenum and Sulfur Precursors for Synthesising MoS2 and Its Composites
4. Electrical and Capacitive Properties for MoS2-Based Electrode Materials in Energy Storage Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Zhu, H.; Ma, Q.; Liu, M.; Wang, B.; Tang, C.; Wang, Y.; Wu, Q.; Wang, X.; Hu, Z. Ultrathin MoS2 nanosheets hybridizing with reduced graphene oxide for high-performance pseudocapacitors. FlatChem 2020, 26, 100212. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef] [Green Version]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Sarmah, D.; Kumar, A. Symmetric Supercapacitors with layer-by-layer Molybdenum disulfide—reduced graphene oxide structures and poly(3,4-ethylenedioxythiophene) nanoparticles nanohybrid electrode. J. Energy Storage 2021, 35, 102289. [Google Scholar] [CrossRef]
- Dutta, S.; De, S. MoS2 Nanosheet/rGO Hybrid: An Electrode Material for High Performance Thin Film Supercapacitor. Mater. Today Proc. 2018, 5, 9771–9775. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Obreja, V.V.N. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material—A review. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-u.-H.A.; Bilal, S. Achieving ultrahigh cycling stability and extended potential window for supercapacitors through asymmetric combination of conductive polymer nanocomposite and activated carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vighnesha, K.M.; Shruthi; Sandhya; Sangeetha, D.N.; Selvakumar, M. Synthesis and characterization of activated carbon/conducting polymer composite electrode for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 914–921. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Cericola, D.; Ruch, P.W.; Kötz, R.; Novák, P.; Wokaun, A. Characterization of bi-material electrodes for electrochemical hybrid energy storage devices. Electrochem. Commun. 2010, 12, 812–815. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xia, Y.Y. A new concept hybrid electrochemical surpercapacitor: Carbon/LiMn2O4 aqueous system. Electrochem. Commun. 2005, 7, 1138–1142. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Yang, Z.M.; Vinodh, R.; Balakrishnan, B.; Rajmohan, R.; Kim, H.J. Rational design of asymmetric aqueous supercapacitor based on NAXMnO2 and N-doped reduced graphene oxide. J. Energy Storage 2020, 28, 101293. [Google Scholar] [CrossRef]
- Chen, B.; Lu, H.; Zhao, N.; Shi, C.; Liu, E.; He, C.; Ma, L. Facile synthesis and electrochemical properties of continuous porous spheres assembled from defect-rich, interlayer-expanded, and few-layered MoS2/C nanosheets for reversible lithium storage. J. Power Sources 2018, 387, 16–23. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, C.; Xu, S.; Zhu, Y.; Xiong, D.; Wang, L.; Chu, P.K. Fabrication and enhanced supercapacitance of hollow nanostructured MoS2 prepared by a CATB-assisted hydrothermal process. Mater. Lett. 2016, 184, 96–99. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2019, 32, 1903826. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Li, J. Graphene and Graphene-like Layered Transition Metal Dichalcogenides in Energy Conversion and Storage. Adv. Mater. 2014, 10, 2165–2181. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, R.; Wang, G. Enhanced Electrochemical Performance of Self-Assembled Nanoflowers of MoS2 Nanosheets as Supercapacitor Electrode Materials. ACS Omega 2019, 4, 15780–15788. [Google Scholar] [CrossRef] [Green Version]
- Manuraj, M.; Kavya Nair, K.V.; Unni, K.N.N.; Rakhi, R.B. High performance supercapacitors based on MoS2 nanostructures with near commercial mass loading. J. Alloys Compd. 2020, 819, 152963. [Google Scholar] [CrossRef]
- Cui, Y.; He, J.; Yuan, F.; Xue, J.; Li, X.; Wang, J. Preparation of MoS2 microspheres through surfactants-assisted hydrothermal synthesis using thioacetamide as reducing agent. Hydrometallurgy 2016, 164, 184–188. [Google Scholar] [CrossRef]
- Tang, G.; Sun, J.; Wei, C.; Wu, K.; Ji, X.; Liu, S.; Tang, H.; Li, C. Synthesis and characterization of flowerlike MoS 2 nanostructures through CTAB-assisted hydrothermal process. Mater. Lett. 2012, 86, 9–12. [Google Scholar] [CrossRef]
- Jayabal, S.; Wu, J.; Chen, J.; Geng, D.; Meng, X. Metallic 1T-MoS2 nanosheets and their composite materials: Preparation, properties and emerging applications. Mater. Today Energy 2018, 10, 264–279. [Google Scholar] [CrossRef]
- Chao, J.; Yang, L.; Liu, J.; Hu, R.; Zhu, M. Sandwiched MoS2/polyaniline nanosheets array vertically aligned on reduced graphene oxide for high performance supercapacitors. Electrochim. Acta 2018, 270, 387–394. [Google Scholar] [CrossRef]
- Wang, X.; Ding, W.; Li, H.; Li, H.; Zhu, S.; Zhu, X.; Dai, J.; Sheng, Z.; Wang, H.; Zhu, X.; et al. Unveiling highly ambient-stable multilayered 1T-MoS2 towards all-solid-state flexible supercapacitors. J. Mater. Chem. A 2019, 7, 19152–19160. [Google Scholar] [CrossRef]
- Murugan, M.; Mohan Kumar, R.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Synthesis and property studies of molybdenum disulfide modified reduced graphene oxide (MoS2-rGO) Nanocomposites for Supercapacitor Applications. J. Nanosci. Nanotechnol. 2017, 17, 5469–5474. [Google Scholar] [CrossRef]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Bello, I.T.; Oladipo, A.O.; Adedokun, O.; Dhlamini, S.M. Recent advances on the preparation and electrochemical analysis of MoS2-based materials for supercapacitor applications: A mini-review. Mater. Today Commun. 2020, 25, 101664. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Sun, Y.; Jiao, Q. Hydrothermal synthesis of MoS2 with different morphology and its performance in thermal battery. J. Power Sources 2018, 395, 318–327. [Google Scholar] [CrossRef]
- Naz, R.; Imtiaz, M.; Liu, Q.; Yao, L.; Abbas, W.; Li, T.; Zada, I.; Yuan, Y.; Chen, W.; Gu, J. Highly defective 1T-MoS2 nanosheets on 3D reduced graphene oxide networks for supercapacitors. Carbon N. Y. 2019, 152, 697–703. [Google Scholar] [CrossRef]
- Kumar, N.A.; Dar, M.A.; Gul, R.; Baek, J.B. Graphene and molybdenum disulfide hybrids: Synthesis and applications. Mater. Today 2015, 18, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Ye, J.; Chen, W.; Wang, J.; Liu, R.; Lee, J.Y. Synthesis of Few-Layer MoS2-Graphene Composites with Superior Electrochemical Lithium-Storage Performance by an Ionic-Liquid-Mediated Hydrothermal Route. ChemElectroChem 2015, 2, 538–546. [Google Scholar] [CrossRef]
- Hongtao, L.; Zichen, X.; Lina, Z.; Zhiqiang, Z.; Li, X. The effects of different surfactants on the morphologies and electrochemical properties of MoS2/reduce graphene oxide composites. Chem. Phys. Lett. 2019, 716, 6–10. [Google Scholar] [CrossRef]
- Peng, W.; Wang, W.; Han, G.; Huang, Y.; Zhang, Y. Fabrication of 3D flower-like MoS2/graphene composite as high-performance electrode for capacitive deionization. Desalination 2020, 473, 114191. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Vaia, R.A.; Ishii, H.; Giannelis, E.P. Synthesis and Properties of Two-Dimensional Nanostructures by Direct Intercalation of Polymer Melts in Layered Silicates. Chem. Mater. 1993, 5, 1694–1696. [Google Scholar] [CrossRef]
- Bang, G.S.; Nam, K.W.; Kim, J.Y.; Shin, J.; Choi, J.W.; Choi, S.Y. Effective liquid-phase exfoliation and sodium ion battery application of MoS2 nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 7084–7089. [Google Scholar] [CrossRef]
- Li, J.; Gao, D.; Wang, J.; Miao, S.; Wang, G.; Bao, X. Ball-milling MoS2/carbon black hybrid material for catalyzing hydrogen evolution reaction in acidic medium. J. Energy Chem. 2015, 24, 608–613. [Google Scholar] [CrossRef]
- Wang, H.; Tran, D.; Moussa, M.; Stanley, N.; Tung, T.T.; Yu, L.; Yap, P.L.; Ding, F.; Qian, J.; Losic, D. Improved preparation of MoS2/graphene composites and their inks for supercapacitors applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114700. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiry, D.; Goswami, A.; Kappera, R.; Silva, C.D.C.C.E.; Kaplan, D.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nat. Chem. 2015, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sheng, C.; Krishnamoorthy, A.; Rajak, P.; Tiwari, S.; Nomura, K.I.; Misawa, M.; Shimojo, F.; Kalia, R.K.; Nakano, A.; et al. Chemical Vapor Deposition Synthesis of MoS2 Layers from the Direct Sulfidation of MoO3 Surfaces Using Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 7494–7503. [Google Scholar] [CrossRef]
- Gao, A.; Zeng, D.; Liu, Q.; Yi, F.; Shu, D.; Cheng, H.; Zhou, X.; Li, S.; Zhang, F. Molecular self-assembly assisted synthesis of carbon nanoparticle-anchored MoS2 nanosheets for high-performance supercapacitors. Electrochim. Acta 2019, 295, 187–194. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Liu, Y.J.; Liu, Y.M.; Wang, H.B.; Gan, T.; Wang, L.L. Layered MoS2-graphene composites for supercapacitor applications with enhanced capacitive performance. Int. J. Hydrog. Energy 2013, 38, 14027–14034. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Xu, H.; Qian, Y.; Yang, J. Enhanced lithium storage performances of hierarchical hollow MoS2 nanoparticles assembled from nanosheets. ACS Appl. Mater. Interfaces 2013, 5, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Sun, H.; Ji, Y.; Li, W.; Wang, X. Three-dimensional assembly of single-layered Mos2. Adv. Mater. 2014, 26, 964–969. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Z.; Gao, Y.; Chen, L.; Lu, M.; Jiao, Z.; Jiang, Y.; Ding, Y.; Cheng, L. Hydrothermal synthesis of layer-controlled MoS2 /graphene composite aerogels for lithium-ion battery anode materials. Appl. Surf. Sci. 2016, 390, 209–215. [Google Scholar] [CrossRef]
- Fan, L.Q.; Liu, G.J.; Zhang, C.Y.; Wu, J.H.; Wei, Y.L. Facile one-step hydrothermal preparation of molybdenum disulfide/carbon composite for use in supercapacitor. Int. J. Hydrog. Energy 2015, 40, 10150–10157. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.X.; Li, H.; Zheng, Y.F.; Xu, Z. De Ionic liquid-assisted hydrothermal synthesis of MoS2 microspheres. Mater. Lett. 2008, 62, 797–799. [Google Scholar] [CrossRef]
- Nagaraju, G.; Tharamani, C.N.; Chandrappa, G.T.; Livage, J. Hydrothermal synthesis of amorphous MoS 2 nanofiber bundles via acidification of ammonium heptamolybdate tetrahydrate. Nanoscale Res. Lett. 2007, 2, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.C.; Zhu, Y.L.; Zhou, Q.W.; Di Zheng, X.; Jiao, Q.J. Fabrication and shape evolution of CoS2 octahedrons for application in supercapacitors. Electrochim. Acta 2014, 136, 550–556. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, S.; Zhang, Y.; Zhang, T.; Zhou, X.; Sun, Y.; Li, T.X.; Fan, H.J.; Shen, G.; Chen, X.; et al. Self-induced uniaxial strain in MoS2 monolayers with local van der waals-stacked interlayer interactions. ACS Nano 2015, 9, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, G.; Zhang, J.; Xu, X. Structural, mechanical and electronic properties of in-plane 1T/2H phase interface of MoS2 heterostructures. AIP Adv. 2015, 5, 097174. [Google Scholar] [CrossRef] [Green Version]
- Baig, M.M.; Pervaiz, E.; Yang, M.; Gul, I.H. High-Performance Supercapacitor Electrode Obtained by Directly Bonding 2D Materials: Hierarchal MoS2 on Reduced Graphene Oxide. Front. Mater. 2020, 7, 580424. [Google Scholar] [CrossRef]
- Nadeem Riaz, K.; Yousaf, N.; Bilal Tahir, M.; Israr, Z.; Iqbal, T. Facile hydrothermal synthesis of 3D flower-like La-MoS2 nanostructure for photocatalytic hydrogen energy production. Int. J. Energy Res. 2019, 43, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon N. Y. 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Ma, L.; Xu, L.M.; Zhou, X.P.; Xu, X.Y. Biopolymer-assisted hydrothermal synthesis of flower-like MoS2 microspheres and their supercapacitive properties. Mater. Lett. 2014, 132, 291–294. [Google Scholar] [CrossRef]
- Thangappan, R.; Kalaiselvam, S.; Elayaperumal, A.; Jayavel, R.; Arivanandhan, M.; Karthikeyan, R.; Hayakawa, Y. Graphene decorated with MoS2 nanosheets: A synergetic energy storage composite electrode for supercapacitor applications. Dalt. Trans. 2016, 45, 2637–2646. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Chen, W.; Chang, K.; Ma, L.; Huang, G.; Chen, D.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2-graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Liu, X.; Ma, L.; Wang, J.; Yang, S. Facile construction of 3D graphene/MoS2 composites as advanced electrode materials for supercapacitors. J. Power Sources 2016, 331, 180–188. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Yang, M.; Qi, Y. Hierarchical hollow MoS2 nanospheres with enhanced electrochemical properties used as an Electrode in Supercapacitor. Electrochim. Acta 2015, 186, 391–396. [Google Scholar] [CrossRef]
- Liu, M.C.; Xu, Y.; Hu, Y.X.; Yang, Q.Q.; Kong, L.B.; Liu, W.W.; Niu, W.J.; Chueh, Y.L. Electrostatically Charged MoS 2 /Graphene Oxide Hybrid Composites for Excellent Electrochemical Energy Storage Devices. ACS Appl. Mater. Interfaces 2018, 10, 35571–35579. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Karuppasamy, K.; Hussain, S.; Kathalingam, A.; Sanmugam, A.; Jung, J.; Kim, H.S. One-pot facile methodology to synthesize MoS2-graphene hybrid nanocomposites for supercapacitors with improved electrochemical capacitance. Compos. Part B Eng. 2019, 161, 555–563. [Google Scholar] [CrossRef]
- Vikraman, D.; Rabani, I.; Hussain, S.; Sundaram, K.; Ramesh, S.; Kim, H.S.; Seo, Y.S.; Jung, J.; Kim, H.S. Mixed-phase MoS2 decorated reduced graphene oxide hybrid composites for efficient symmetric supercapacitors. Int. J. Energy Res. 2021, 45, 9193–9209. [Google Scholar] [CrossRef]
- Sabeeh, H.; Zulfiqar, S.; Aadil, M.; Shahid, M.; Shakir, I.; Khan, M.A.; Warsi, M.F. Flake-like MoS2 nano-architecture and its nanocomposite with reduced Graphene Oxide for hybrid supercapacitors applications. Ceram. Int. 2020, 46, 21064–21072. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, A.; Yang, B.; Wang, D.; Zhang, Z. Flower-like MoS 2 onto nitrogen-doped 3D graphene composite with active material for supercapacitor electrodes. Mater. Lett. 2019, 240, 258–261. [Google Scholar] [CrossRef]
- Yang, M.H.; Jeong, J.M.; Huh, Y.S.; Choi, B.G. High-performance supercapacitor based on three-dimensional MoS2/graphene aerogel composites. Compos. Sci. Technol. 2015, 121, 123–128. [Google Scholar] [CrossRef]
- Chen, M.; Dai, Y.; Wang, J.; Wang, Q.; Wang, Y.; Cheng, X.; Yan, X. Smart combination of three-dimensional-flower-like MoS2nanospheres/interconnected carbon nanotubes for application in supercapacitor with enhanced electrochemical performance. J. Alloys Compd. 2017, 696, 900–906. [Google Scholar] [CrossRef]
- Gupta, H.; Chakrabarti, S.; Mothkuri, S.; Padya, B.; Rao, T.N.; Jain, P.K. High performance supercapacitor based on 2D-MoS2 nanostructures. Mater. Today Proc. 2018, 26, 20–24. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Liu, Y.J.; Wang, H.B.; Liu, Y.M.; Wang, L.L. Synthesis of polyaniline/2-dimensional graphene analog MoS2composites for high-performance supercapacitor. Electrochim. Acta 2013, 109, 587–594. [Google Scholar] [CrossRef]







| Composite | Precursors | Surfactant | Synthesis Method | Morphology | Synthesis Conditions | Specific Capacitance (F·g−1) | Specific Surface Area (m2·g−1) | Cycling Stability | Collectors | Electrode Test System | Electrolyte | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/MoS2 | Na2MoO4 and C3H7NO2S | β-cyclodextrin | Hydrothermal | Flower-like | 200 °C for 12 h | 394 at 5 mV/s | 6.36 | 60.68% after 2000 cycles at 40 mV/s | Stainless Steel | Three electrode system | 1 M Na2SO4 | [53] |
| MoS2/rGO | (NH4)6Mo7O24 and CH4N2S | - | One pot reaction | Nanospheres | 90 °C for 8 h | 275 at 1 A·g−1 | 9.02 | 97% after 5000 cycles at 1 A·g−1 | Ni foam | Two electrode system | 6 M KOH | [75] |
| MoS2/G | Na2MoO4 and CH4N2S | - | Hydrothermal | 3D spheres | 180 °C for 36 h | 243 at 1 A·g−1 | 102.8 | 92.3% after 1000 cycles at 1 A·g−1 | Stainless Steel | Three electrode system | 1 M Na2SO4 | [54] |
| MoS2/rGO | (NH4)6Mo7O24 and C2H5NS | Citric Acid | Hydrothermal | Wrinkly paper like | 180 °C for 24 h | 334 at 0.5 mV/s | - | 90% after 500 cycles at 10 mV/s | Platinum strip | Three electrode system | 1 M LiClO4 | [33] |
| MoS2/rGO | (NH4)6Mo7O24 and C3H7NO2S | - | Hydrothermal | Wrinkly paper like | 180 °C for 20 h | 361 at 5 mV/s | - | 92% after 10,000 cycles | Carbon paper | Two & Three electrode system | 1 M H2SO4 | [1] |
| MoS2/Ni foam | Na2MoO4 and CH4N2S | CTAB | Hydrothermal | Hollow nanostructure | 160–200 °C for 24 h | 160.1 at 1 A·g−1 | - | 94.8% after 3000 cycles at 1 A·g−1 | Ni foam | Three electrode system | 1 M Na2SO4 | [21] |
| MoS2 | Na2MoO4 and C2H5NS | - | Hydrothermal | Brush like | 180 °C for 36 h | 244 at 1 A·g−1 | 18.45 | 92% after 9000 cycles at 5 A·g−1 | Ni foam | Three electrode system | 1 M KOH | [27] |
| MoS2/G | (NH4)6Mo7O24 and CH4N2S | - | One pot reaction | Nanosheet grains | 50 °C for 6 h | 756 at 0.5 A·g−1 | - | 88% after 10,000 cycles at 0.5 A·g−1 | Stainless steel | Three electrode system | 1 M KOH | [74] |
| MoS2 | Na2MoO4 and C3H7NO2S | - | Hydrothermal | Nanospheres | 220 °C for 24 h | 142 at 0.59 A·g−1 | - | 92.9% after 1000 cycles at 0.59 A·g−1 | Ni foam | Three electrode system | 1 M KCl | [72] |
| MoS2/PANI/ rGO | (NH4)6Mo7O24 and CH4N2S | - | Hydrothermal | Flower-like | 200 °C for 48 h | 330.7 at 10 A·g−1 | 85.3 | 81.9 % after 40,000 cycles at 10 A·g−1 | Graphite paper | Three electrode system | 1 M H2SO4 | [31] |
| MoS2/rGO | MoO3·H2O and C2H5NS | Urea | Hydrothermal | Flake-like | 210 °C for 18 h | 441 at 1 A·g−1 | 163.6 | 84.2% after 1000 cycles | ITO | Three electrode system | 1 M Na2SO4 | [76] |
| MoS2/N-doped 3D Graphene | (NH4)6Mo7O24 and CH4N2S | - | Hydrothermal | Flower-like | 200 °C for 24 h | 301.2 at 0.2 A·g−1 | - | 82% after 1000 cycles at 1 A·g−1 | Glassy carbon | Three electrode system | 1 M Na2SO4 | [77] |
| MoS2/G aerogel | Li intercalation | - | Hydrothermal | 3D porous network | 180 °C for 12 h | 268 at 0.5 A·g−1 | 149.3 | 93% after 1000 cycles | Ti foil | Three electrode system | 1 M Na2SO4 | [78] |
| MoS2/CNT | MoO3 and KSCN | SDBS | Hydrothermal | Flower-like | 220 °C for 24 h | 74.05 at 2 A·g−1 | 92.25 | 80.8% after 1000 cycles at 1 A·g−1 | Ni foam | Three electrode system | 1 M Na2SO4 | [79] |
| MoS2 | (NH4)6Mo7O24 and CH4N2S | - | Hydrothermal | Flower-like | 220 °C for 24 h | 255.65 at 0.25 A·g−1 | - | 70% | Graphite foil | Two electrode system | 3 M KOH | [80] |
| MoS2/rGO | (NH4)6Mo7O24 and CH4N2S | PVP | Hydrothermal | Flower-like | 180 °C for 16 h | 850 at 1 A·g−1 | 391 | 95.33% after 10,000 cycles at 2 A·g−1 | Ni foam | Three electrode system | 1 M KCl | [65] |
| MoS2/rGO | (NH4)6Mo7O24 and CH4N2S | - | Hydrothermal | Flower-like | 220 °C for 6 h | 442 at 1 A·g−1 | 29.27 | 90.3% after 1000 cycles at 5 A·g−1 | Glassy carbon | Three electrode system | 1 M H2SO4 | [37] |
| MoS2/3D-G | Na2MoO4 and CH4N2S | - | Hydrothermal | Nanoflowers | 200 °C for 24 h | 410 at 1 A·g−1 | 165.7 | 90.3% after 10,000 cycles at 2 A·g−1 | Ni foam | Three electrode system | 1 M Na2SO4 | [71] |
| MoS2/PANI | MoS2 powder and aniline | - | In situ chemical process | Flower-like | 12 h ice bath polymerization | 575 at 1 A·g−1 | - | >98% after 500 cycles at 1 A·g−1 | Stainless steel | Three electrode system | 1 M H2SO4 | [81] |
| MoS2/rGO | Na2MoO4 and C2H5NS | CTAB | Hydrothermal | Nanoparticles | 180 °C for 12 h | 1022 at 0.3 A·g−1 | 133 | - | - | - | - | [40] |
| MoS2 | MoO3 and C2H5NS | - | Hydrothermal | Nanoflowers | 200 °C for 12 h | 1120 | 54.7 | 96% after 2000 cycles at 10 A·g−1 | Ni foam | Three electrode system | 3 M KOH | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salagean, C.A.; Costinas, C.; Cotet, L.C.; Baia, L. Insights into the Influence of Key Preparation Parameters on the Performance of MoS2/Graphene Oxide Composites as Active Materials in Supercapacitors. Catalysts 2021, 11, 1553. https://doi.org/10.3390/catal11121553
Salagean CA, Costinas C, Cotet LC, Baia L. Insights into the Influence of Key Preparation Parameters on the Performance of MoS2/Graphene Oxide Composites as Active Materials in Supercapacitors. Catalysts. 2021; 11(12):1553. https://doi.org/10.3390/catal11121553
Chicago/Turabian StyleSalagean, Catalin Alexandru, Codrut Costinas, Liviu Cosmin Cotet, and Lucian Baia. 2021. "Insights into the Influence of Key Preparation Parameters on the Performance of MoS2/Graphene Oxide Composites as Active Materials in Supercapacitors" Catalysts 11, no. 12: 1553. https://doi.org/10.3390/catal11121553
APA StyleSalagean, C. A., Costinas, C., Cotet, L. C., & Baia, L. (2021). Insights into the Influence of Key Preparation Parameters on the Performance of MoS2/Graphene Oxide Composites as Active Materials in Supercapacitors. Catalysts, 11(12), 1553. https://doi.org/10.3390/catal11121553