Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts
Abstract
:1. Introduction
2. Results and Discussion
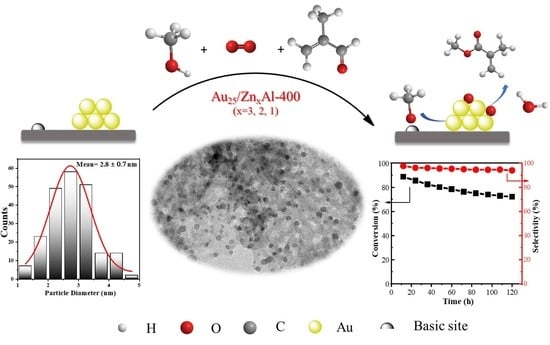
2.1. Catalytic Performances
2.2. Structure of the Catalysts
2.3. Electronic Property of the Catalyst
2.4. The Basic Property of the Catalyst
2.5. The Active Sites and Reaction Mechanism
2.6. Substrate Universality of the Catalyst
2.7. Stability of Catalysts in Fixed Bed Reactor
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Supported Gold Clusters
3.3. Preparation of Supported Gold Catalysts with DP Method
3.4. Catalytic Test
3.5. Stability Test
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagai, K. New developments in the production of methyl methacrylate. Appl. Catal. A Gen. 2001, 221, 367–377. [Google Scholar] [CrossRef]
- Global Industry Analysts Inc, Methyl Methacrylate (MMA)—Global Market Trajectory Analytics. Available online: https://www.researchandmarkets.com/categories/chemicals-materials (accessed on 5 December 2020).
- Wang, B.; Li, H.; Zhu, J.; Sun, W.; Chen, S. Preparation and characterization of mono-/multi-metallic hydrophobic catalysts for the oxidative esterification of methacrolein to methyl methacrylate. J. Mol. Catal. A Chem. 2013, 379, 322–326. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Zuo, C.; Yin, D.; Wang, L.; Zheng, Y.; Huang, H.; Fu, Z.; Wang, M. Ionic-Liquid-Modified Porous Au/CeMnOx Nanorods for Methyl Methacrylate (MMA) Synthesis via Direct Oxidative Esterification. Chemnanomat 2019, 5, 1361–1366. [Google Scholar] [CrossRef]
- Diao, Y.; He, H.; Yang, P.; Wang, L.; Zhang, S. Optimizing the structure of supported Pd catalyst for direct oxidative esterification of methacrolein with methanol. Chem. Eng. Sci. 2015, 135, 128–136. [Google Scholar] [CrossRef]
- Diao, Y.; Yang, P.; Yan, R.; Jiang, L.; Wang, L.; Zhang, H.; Li, C.; Li, Z.; Zhang, S. Deactivation and regeneration of the supported bimetallic Pd–Pb catalyst in direct oxidative esterification of methacrolein with methanol. Appl. Catal. B Environ. 2013, 142, 329–336. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Dubois, J.-L.; Cavani, F.; Rostamizadeh, M.; Patience, G.S. Catalysis for the synthesis of methacrylic acid and methyl methacrylate. Chem. Soc. Rev. 2018, 47, 7703–7738. [Google Scholar] [CrossRef] [PubMed]
- Yamamatsu, S.; Yamaguchi, T.; Yokota, K.; Nagano, O.; Chono, M.; Aoshima, A. Development of Catalyst Technology for Producing Methyl Methacrylate (MMA) by Direct Methyl Esterification. Catal. Surv. Asia 2010, 14, 124–131. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous Gold-Based Catalysis for Green Chemistry: Low-Temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yang, S.M. Complete oxidation of 2-propanol over gold-based catalysts supported on metal oxides. Appl. Catal. A Gen. 2008, 334, 92–99. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nat. Cell Biol. 2008, 454, 981–983. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-Gold Catalysis in Fine Chemical Synthesis. Chem. Rev. 2011, 112, 2467–2505. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, H.; Liu, X.Y.; Wang, A.; Liu, C.; Zhang, T. Effective removal of the protective ligands from Au nanoclusters by ambient pressure nonthermal plasma treatment for CO oxidation. Chin. J. Catal. 2018, 39, 929–936. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y.; et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Yamaguchi, T.; Matsushita, K.; Iitsuka, C.; Miura, J.; Akaogi, T.; Ishida, H. Aerobic Oxidative Esterification of Aldehydes with Alcohols by Gold–Nickel Oxide Nanoparticle Catalysts with a Core–Shell Structure. ACS Catal. 2013, 3, 1845–1849. [Google Scholar] [CrossRef]
- Wan, X.; Deng, W.; Zhang, Q.; Wang, Y. Magnesia-supported gold nanoparticles as efficient catalysts for oxidative esterification of aldehydes or alcohols with methanol to methyl esters. Catal. Today 2014, 233, 147–154. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yan, R.; Han, J.; Zhang, S. Gold nanoparticles supported on Ce–Zr oxides for the oxidative esterification of aldehydes to esters. Catal. Sci. Technol. 2015, 5, 3682–3692. [Google Scholar] [CrossRef]
- Zuo, C.; Tian, Y.; Zheng, Y.; Wang, L.; Fu, Z.; Jiao, T.; Wang, M.; Huang, H.; Li, Y. One step oxidative esterification of methacrolein with methanol over Au-CeO2/γ-Al2O3 catalysts. Catal. Commun. 2019, 124, 51–55. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Zheng, Y.; Wang, M.; Zuo, C.; Huang, H.; Yin, D.; Fu, Z.; Tan, J.; Zhou, Z. Nano-Au/MCeOx catalysts for the direct oxidative esterification of methylacrolein to methyl esters. Ind. Eng. Chem. Res. 2019, 58, 19397–19405. [Google Scholar] [CrossRef]
- Paul, B.; Khatun, R.; Sharma, S.K.; Adak, S.; Singh, G.; Das, D.; Siddiqui, N.; Bhandari, S.; Joshi, V.; Sasaki, T.; et al. Fabrication of Au Nanoparticles Supported on One-Dimensional La2O3 Nanorods for Selective Esterification of Methacrolein to Methyl Methacrylate with Molecular Oxygen. ACS Sustain. Chem. Eng. 2019, 7, 3982–3994. [Google Scholar] [CrossRef]
- Gao, J.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Li, F. Oxidative Esterification of Methacrolein to Methyl Methacrylate over Gold Nanoparticles on Hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Tan, Y.; Ding, Y. Research progress on the production technology and noble metal catalyst for synthesis of methyl methacrylate. Chem. Ind. Eng. Prog. 2021, 40, 183–194. [Google Scholar]
- Seftel, E.; Popovici, E.; Mertens, M.; De Witte, K.; Van Tendeloo, G.; Cool, P.; VanSant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Aliç, F.; Verberckmoes, A.; Van Der Voort, P. Tuning the acidic–basic properties by Zn-substitution in Mg–Al hydrotalcites as optimal catalysts for the aldol condensation reaction. J. Mater. Sci. 2016, 52, 628–642. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Luo, W.; Meng, Y.; Zhou, X.; Pan, G.; Ni, Z.; Xia, S. Visible-Light-Promoted Selective Hydrogenation of Crotonaldehyde by Au Supported ZnAl-Layered Double Hydroxides: Catalytic Property, Kinetics, and Mechanism Investigation. J. Phys. Chem. C 2018, 122, 17358–17369. [Google Scholar] [CrossRef]
- Fang, W.; Chen, J.; Zhang, Q.; Deng, W.; Wang, Y. Hydrotalcite-Supported Gold Catalyst for the Oxidant-Free Dehydrogenation of Benzyl Alcohol: Studies on Support and Gold Size Effects. Chem. A Eur. J. 2010, 17, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, M.; Bagnasco, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Ramis, G.; Busca, G. Production of hydrogen from oxidative steam reforming of methanolII. Catalytic activity and reaction mechanism on Cu/ZnO/Al2O3 hydrotalcite-derived catalysts. J. Catal. 2004, 228, 56–65. [Google Scholar] [CrossRef]
- Kirm, I.; Medina, F.; Rodríguez, X.; Cesteros, Y.; Salagre, P.; Sueiras, J. Epoxidation of styrene with hydrogen peroxide using hydrotalcites as heterogeneous catalysts. Appl. Catal. A Gen. 2004, 272, 175–185. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Zhang, L.; Wang, A.; Li, L.; Pan, X.; Miao, S.; Haruta, M.; Wei, H.; Wang, H.; et al. ZnAl-Hydrotalcite-Supported Au25 Nanoclusters as Precatalysts for Chemoselective Hydrogenation of 3-Nitrostyrene. Angew. Chem. Int. Ed. 2017, 56, 2709–2713. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.Y.; Li, L.; Kang, L.; Wang, A.; Zhang, T. Effects of divalent metal ions of hydrotalcites on catalytic behavior of supported gold nanocatalysts for chemoselective hydrogenation of 3-nitrostyrene. J. Catal. 2018, 364, 174–182. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.Y.; Zhang, L.; Liu, F.; Wang, A.; Zhang, T. Producing of cinnamyl alcohol from cinnamaldehyde over supported gold nanocatalyst. Chin. J. Catal. 2021, 42, 470–481. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Włodarczyk-Stasiak, M.; Jamroz, J. Specific surface area and porosity of starch extrudates determined from nitrogen adsorption data. J. Food Eng. 2009, 93, 379–385. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Knözinger, H.; Hadjiivanov, K.; Gates, B.C. Characterization of the Oxidation States of Supported Gold Species by IR Spectroscopy of Adsorbed CO. Chem. Ing. Tech. 2007, 79, 795–806. [Google Scholar] [CrossRef]
- George, G.; Saravanakumar, M. Correction to: Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: Application on crystal violet and malachite green dye adsorption—Isotherm, kinetics and Box-Behnken design. Environ. Sci. Pollut. Res. 2018, 25, 30255–30256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, J.J.; Aguilar-Martínez, O.; Piña-Pérez, Y.; Pérez-Hernández, R.; Santolalla-Vargas, C.E.; Gómez, R.; Tzompantzi, F. Efficient ZnS–ZnO/ZnAl-LDH composite for H2 production by photocatalysis. Renew. Energy 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Hua, Z.; Lang, J.; Song, Y.; Zhang, Q.; Han, Q.; Fan, H.; Gao, M.; Li, X.; Fan, H. Eco-friendly nanostructured Zn–Al layered double hydroxide photocatalysts with enhanced photocatalytic activity. CrystEngComm 2019, 21, 4607–4619. [Google Scholar] [CrossRef]
- Trimpalis, A.; Giannakakis, G.; Cao, S.; Flytzani-Stephanopoulos, M. NiAu single atom alloys for the selective oxidation of methacrolein with methanol to methyl methacrylate. Catal. Today 2020, 355, 804–814. [Google Scholar] [CrossRef]
- Li, J.; Chao, H.; Kai, T.; Hao, X.; Zhu, Z.; Hu, Z. CO2 atmosphere-enhanced methanol aromatization over the NiO-HZSM-5 catalyst. Rsc. Adv. 2014, 4, 44377–44385. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Taketoshi, A.; Haruta, M. ChemInform Abstract: Size- and Structure-Specificity in Catalysis by Gold Clusters. Chem. Lett. 2014, 45, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Liu, X.; Haubrich, J.; Friend, C.M. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol. Nat. Chem. 2009, 2, 61–65. [Google Scholar] [CrossRef] [PubMed]










| Entry | Catalysts | Conversion (%) a | Selectivity (%) a | TOF (h−1) b | ||
|---|---|---|---|---|---|---|
| MMA | ACE | Others | ||||
| 1 | Au25/Zn3Al-400 | 84.9 | 95.1 | 0.7 | 4.2 | 1734 |
| 2 | Au25/Zn2Al-400 | 93.1 | 94.8 | 0.8 | 4.4 | 1626 |
| 3 | Au25/Zn1Al-400 | 90.1 | 92.0 | 0.5 | 7.5 | 1675 |
| 4 | Au/Zn2Al-400 | 57.1 | 94.1 | 3.4 | 2.5 | 684 |
| 5 | Zn3Al-400 | 6.4 | 0 | 99.0 | - | - |
| 6 | Zn2Al-400 | 13.2 | 0 | 94.8 | - | - |
| 7 | Zn1Al-400 | 16.9 | 0 | 91.0 | - | - |
| 8 | Blank | 0 | - | - | - | - |
| Entry | Catalyst | Loadings of Gold (wt %) a | Surface Area (m2/g) b | Pore Volume (cm3/g) c | Half Pore Width (nm) d | Particle Size of Gold (nm) e | Total Basicity (µmol CO2/gcat) f |
|---|---|---|---|---|---|---|---|
| 1 | Au25/Zn3Al-400 | 1.27 | 49.1 | 0.24 | 1.71 | 2.8 | 640 |
| 2 | Au25/Zn2Al-400 | 1.18 | 60.5 | 0.14 | 1.53 | 2.5 | 500 |
| 3 | Au25/Zn1Al-400 | 1.32 | 80.9 | 0.27 | 1.71 | 2.5 | 430 |
| 4 | Au/Zn2Al-400 | 1.32 | 71.0 | 0.08 | 1.90 | 2.8 | 540 |
| Entry | Aldehyde | Alcohol | Product | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|
| 1 |  | Methanol |  | 69.4 | 92.0 |
| 2 |  | Ethanol |  | 53.9 | 64.4 |
| 3 |  | Methanol |  | 87.8 | 98.9 |
| 4 |  | Ethanol |  | 91.2 | 97.9 |
| 5 |  | Methanol |  | 87.8 | 95.2 |
| 6 |  | Ethanol |  | 93.6 | 91.9 |
| 7 |  | Methanol |  | 45.2 | 75.4 |
| 8 |  | Ethanol |  | 32.4 | 90.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tan, Y.; Chen, X.; Yang, W.; Huang, C.; Li, J.; Ding, Y. Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts. Catalysts 2021, 11, 162. https://doi.org/10.3390/catal11020162
Li H, Tan Y, Chen X, Yang W, Huang C, Li J, Ding Y. Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts. Catalysts. 2021; 11(2):162. https://doi.org/10.3390/catal11020162
Chicago/Turabian StyleLi, Huayin, Yuan Tan, Xingkun Chen, Wenshao Yang, Chuanqi Huang, Jie Li, and Yunjie Ding. 2021. "Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts" Catalysts 11, no. 2: 162. https://doi.org/10.3390/catal11020162
APA StyleLi, H., Tan, Y., Chen, X., Yang, W., Huang, C., Li, J., & Ding, Y. (2021). Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts. Catalysts, 11(2), 162. https://doi.org/10.3390/catal11020162