Cu-IM-5 as the Catalyst for Selective Catalytic Reduction of NOx with NH3: Role of Cu Species and Reaction Mechanism
Abstract
:1. Introduction
2. Results
2.1. Structure Characterization
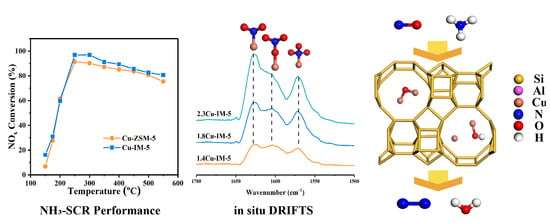
2.2. NH3-SCR Performance over Cu-IM-5
2.3. Cu Species in Cu-IM-5
2.4. Reaction Mechanisms
3. Discussion
4. Materials and Methods
4.1. Synthesis of Cu-IM-5
4.2. Characterization of Cu-IM-5
4.3. NH3-SCR Test
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chavannavar, P. Exhaust Aftertreatment System and Method. U.S. Patent No 9,132,386, 3 April 2014. [Google Scholar]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y.; Zhang, Z.; Zhang, C.; Fu, G.; Yi, X.; Huang, Q.; Yang, F.; Liang, W.; Zheng, A.; et al. Remarkable performance of selective catalytic reduction of NOx by ammonia over copper-exchanged SSZ-52 catalysts. Appl. Catal. B Environ. 2021, 283, 9641. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective Transformation of Various Nitrogen-Containing Exhaust Gases toward N2 over Zeolite Catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Z.; Huang, J.; Xia, Q.; Wu, Y.; Chen, H.; Fang, Y. Development of Iron Encapsulated Hollow Beta Zeolites for Ammonia Selective Catalytic Reduction. Ind. Eng. Chem. Res. 2019, 58, 2914–2923. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, K.; Liao, Z.; Chen, P.; Chen, D.; Wu, Y.; Xia, Q.; Xi, H.; Dong, J. Fe-Encapsulated ZSM-5 Zeolite with Nanosheet-Assembled Structure for the Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2020, 59, 8592–8600. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Mao, W.; Chen, H.; Han, L.; Zhu, K.; Zhou, X. Pickering emulsion mediated crystallization of hierarchical zeolite SSZ-13 with enhanced NH3 selective catalytic reduction performance. Micropor. Mesopor. Mater. 2019, 285, 202–214. [Google Scholar] [CrossRef]
- Iwamoto, M.; Furukawa, H.; Mine, Y.; Uemura, F.; Mikuriya, S.I.; Kagawa, S.J. Copper(II) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. Chem. Soc. Chem. Commun. 1986, 1272–1273. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Baerlocher, C.; Gramm, F.; Massüger, L.; McCusker, L.B.; He, Z.; Hovmöller, S.; Zou, X. Structure of the Polycrystalline Zeolite Catalyst IM-5 Solved by Enhanced Charge Flipping. Science 2007, 315, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Chen, J.; Li, W.; Wang, H.; Deng, K.; Vinokurov, V.A.; Huang, W. Hydrodeoxygenation of bio-derived anisole to cyclohexane over bi-functional IM-5 zeolite supported Ni catalysts. Sustain. Energy Fuels 2019, 3, 3462–3472. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, F.; Chen, J.; Shi, Y.; Cao, H.; Ning, P.; Sun, S.; Xie, Y. Conversion of phenol to cyclohexane in the aqueous phase over Ni/zeolite bi-functional catalysts. Front. Chem. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Vennestrøm, P.N.R.; Janssens, T.V.W.; Kustov, A.; Grill, M.; Molina, P.A.; Lundegaard, L.F.; Tiruvalam, R.R.; Concepción, P.; Corma, A.J. Influence of lattice stability on hydrothermal deactivation of Cu-ZSM-5 and Cu-IM-5 zeolites for selective catalytic reduction of NOx by NH3. J. Catal. 2014, 309, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Moretti, G.; Ferraris, G.; Fierro, G.; Jacono, M.L.; Morpurgo, S.; Faticanti, M.J. Dimeric Cu(I) species in Cu-ZSM-5 catalysts: The active sites for the NO decomposition. J. Catal. 2005, 232, 476–487. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Nature of active species in copper-based catalysts and their chemistry of transformation of nitrogen oxides. Appl. Catal. Gen 1995, 132, 179–259. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Janas, J.; Gurgul, J.; Socha, R.P.; Shishido, T.; Che, M. Do Cu(II) ions need Al atoms in their environment to make CuSiBEA active in the SCR of NO by ethanol or propane? A spectroscopy and catalysis study. Appl. Catal. B Environ. 2009, 85, 131–138. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and Evaluation of Cu-SAPO-34 Catalysts for Ammonia Selective Catalytic Reduction. 1. Aqueous Solution Ion Exchange. ACS Catal. 2013, 3, 2083–2093. [Google Scholar] [CrossRef]
- Liu, L.L.; Liu, L.; Cao, Y.; Wang, J.; Si, R.; Gao, F.; Dong, L. Controlling Dynamic Structural Transformation of Atomically Dispersed CuOx Species and Influence on Their Catalytic Performances. ACS Catal. 2019, 9, 9840–9851. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, J.H.; Burton, S.D.; Lipton, A.S.; Peden, C.H.F.; Szanyi, J. A Common Intermediate for N2 Formation in Enzymes and Zeolites: Side-On Cu–Nitrosyl Complexes. Angew. Chem. Int. Ed. 2013, 52, 9985–9989. [Google Scholar] [CrossRef] [PubMed]
- Szanyi, J.; Kwak, J.H.; Zhu, H.; Peden, C.H.F. Characterization of Cu-SSZ-13 NH3 SCR catalysts: An in situ FTIR study. Phys. Chem. Chem. Phys. 2013, 15, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Long, R.Q.; Yang, R.T. Reaction Mechanism of Selective Catalytic Reduction of NO with NH3 over Fe-ZSM-5 Catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yamazaki, K.; Banno, K.; Shinjoh, H.J. Characterization of Fe/ZSM-5 DeNOx catalysts prepared by different methods: Relationships between active Fe sites and NH3-SCR performance. J. Catal. 2008, 260, 205–216. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W.J. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R.; Liu, N. Economical way to synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOx by ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef] [PubMed]
- Nanba, T.; Masukawa, S.; Ogata, A.; Uchisawa, J.; Obuchi, A. Active sites of Cu-ZSM-5 for the decomposition of acrylonitrile. Appl. Catal. B Environ. 2005, 61, 288–296. [Google Scholar] [CrossRef]
- Occhiuzzi, M.; Fierro, G.; Ferraris, G.; Moretti, G. Unusual Complete Reduction of Cu2+ Species in Cu-ZSM-5 Zeolites under Vacuum Treatment at High Temperature. Chem. Mater. 2012, 24, 2022–2031. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Peng, H.; Mi, Y.; Liang, J.; Liu, W.; Wang, X.; Song, G.; Wu, P.; Liu, F.J. One-pot synthesis of layered mesoporous ZSM-5 plus Cu ion-exchange: Enhanced NH3-SCR performance on Cu-ZSM-5 with hierarchical pore structures. Hazard. Mater. 2020, 385, 1593. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Yu, Y.; Shan, W.; Shi, X.; He, H. Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized cu-zeolites for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 266, 8655. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Washton, N.M.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Alkali and Alkaline Earth Cocations on the Activity and Hydrothermal Stability of Cu/SSZ-13 NH3-SCR Catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Kamasamudram, K.; Epling, W.S. In Situ-DRIFTS Study of Selective Catalytic Reduction of NOx by NH3 over Cu-Exchanged SAPO-34. ACS Catal. 2013, 3, 871–881. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2014, 156–157, 428–437. [Google Scholar] [CrossRef]
- Olsson, L.; Sjövall, H.; Blint, R.J. Detailed kinetic modeling of NOx adsorption and NO oxidation over Cu-ZSM-5. Appl. Catal. B Environ. 2009, 87, 200–210. [Google Scholar] [CrossRef]
- Zhu, H.; Kwak, J.H.; Peden, C.; Szanyi, J. In situ DRIFTS-MS studies on the oxidation of adsorbed NH3 by NOx over a Cu-SSZ-13 zeolite. Catal. Today 2013, 205, 16–23. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. Enhanced hydrothermal stability of Cu-ZSM-5 catalyst via surface modification in the selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 375, 186–195. [Google Scholar] [CrossRef]
- Sjövall, H.; Fridell, E.; Blint, R.J.; Olsson, L. Identification of adsorbed species on Cu-ZSM-5 under NH3 SCR conditions. Top. Catal. 2007, 42, 113–117. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]












| Sample | Si/Al (ICP) | Framework Si/Al 1 | Cu Contents wt.% | Cu/2Al | EPR Signal Intensity (g┴) | SBET m2/g | Pore Volume cm³/g |
|---|---|---|---|---|---|---|---|
| IM-5 | 16.5 | 13.9 | 0 | 0 | 0 | 354.9 | 0.375 |
| 1.4Cu-IM-5 | 16.8 | 12.8 | 1.4 | 0.56 | 6.4 × 106 | 347.7 | 0.370 |
| 1.8Cu-IM-5 | 17.2 | 12.5 | 1.8 | 0.80 | 3.2 × 106 | 336.3 | 0.358 |
| 2.3Cu-IM-5 | 16.7 | 12.3 | 2.3 | 0.90 | 2.1 × 106 | 328.9 | 0.345 |
| Samples | [Cu(OH)]+ | Cu–O–Cu | Cu2+ |
|---|---|---|---|
| 1.4Cu-IM-5 | 15.2 | 23.7 | 61.1 |
| 1.8Cu-IM-5 | 13.0 | 49.7 | 37.3 |
| 2.3Cu-IM-5 | 12.1 | 56.1 | 31.8 |
| Sample | Fraction of Different Peaks | ||
|---|---|---|---|
| Weak Acid | Cu Species | Strong Acid | |
| IM-5 | 0.407 | - | 0.593 |
| 1.4Cu-IM-5 | 0.446 | 0.172 | 0.381 |
| 1.8Cu-IM-5 | 0.483 | 0.192 | 0.324 |
| Wave Number (cm−1) | Group | Appear (a) and Disappear (d) Time (min) | |
| Figure 11a | Figure 11b | ||
| 3610 | Zeo–OH group | - | - |
| 3275 | N–H stretching of NH4+ on BA | - | - |
| 3181 and 3338 | N–H stretching of NH3 on LA | - | - |
| 1618 | N–H bending of NH3 on LA | 5 (d) | 8 (a) |
| 1480 | N–H bending of NH4+ on BA | 15 (d) | 5 (a) |
| 1625 | NO2 | 5 (a) | 10 (d) |
| 1615 and 1597 | monodentate nitrates | 5 (a) | 10 (d) |
| 1575 | bidentate nitrates | 5 (a) | 15 (d) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, G.; Chen, J.; Liang, Y.; Li, R.; Yang, X.; Jiang, J. Cu-IM-5 as the Catalyst for Selective Catalytic Reduction of NOx with NH3: Role of Cu Species and Reaction Mechanism. Catalysts 2021, 11, 221. https://doi.org/10.3390/catal11020221
Fu G, Chen J, Liang Y, Li R, Yang X, Jiang J. Cu-IM-5 as the Catalyst for Selective Catalytic Reduction of NOx with NH3: Role of Cu Species and Reaction Mechanism. Catalysts. 2021; 11(2):221. https://doi.org/10.3390/catal11020221
Chicago/Turabian StyleFu, Guangying, Junwen Chen, Yuqian Liang, Rui Li, Xiaobo Yang, and Jiuxing Jiang. 2021. "Cu-IM-5 as the Catalyst for Selective Catalytic Reduction of NOx with NH3: Role of Cu Species and Reaction Mechanism" Catalysts 11, no. 2: 221. https://doi.org/10.3390/catal11020221
APA StyleFu, G., Chen, J., Liang, Y., Li, R., Yang, X., & Jiang, J. (2021). Cu-IM-5 as the Catalyst for Selective Catalytic Reduction of NOx with NH3: Role of Cu Species and Reaction Mechanism. Catalysts, 11(2), 221. https://doi.org/10.3390/catal11020221